More Information
Submitted: April 28, 2025 | Approved: May 08, 2025 | Published: May 09, 2025
How to cite this article: Ranjan N, Ahmed A, Woods J, Selvaraj R, Patel M, Dalati Y, et al. Sotatercept in the Treatment of Pulmonary Arterial Hypertension: A Comprehensive Narrative Review of Mechanism, Efficacy and Future Directions. J Pulmonol Respir Res. 2025; 9(1): 014-022. Available from:
https://dx.doi.org/10.29328/journal.jprr.1001068
DOI: 10.29328/journal.jprr.1001068
Copyright license: © 2025 Ranjan N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Sotatercept; Pulmonary arterial hypertension; Activin receptor; 6-minute walk distance; Pulmonary vascular resistance; TGF-β signaling
Sotatercept in the Treatment of Pulmonary Arterial Hypertension: A Comprehensive Narrative Review of Mechanism, Efficacy and Future Directions
Niroshan Ranjan1*, Ahmed Ahmed1, Jordana Woods1, Ramaneshwar Selvaraj1, Mihir Patel1, Yaman Dalati2,3, Vidushan Sabanathan4,5 and Thanujan Thangadurai1
1Henry Ford Allegiance Hospital, Jackson, MI, USA
2Wayne State University, Internal Medicine Residency Program, Detroit, MI, USA
3Henry Ford Detroit Hospital, Detroit, MI, USA
4Medical Student, American University of the Caribbean School of Medicine, USA
5Good Samaritan University Hospital, West Islip, NY, USA
*Address for Correspondence: Niroshan Ranjan, MD, Henry Ford Allegiance Hospital, 1909 Apt 1 Springport Rd, Jackson, MI, 49202, USA, Email: [email protected]
Rationale: Pulmonary Arterial Hypertension (PAH) is a progressive vascular remodeling disease with elevated pulmonary vascular resistance that is lethal. While therapeutic progress was recently made with endothelin, nitric oxide, and prostacyclin pathway-based therapy for the treatment of PAH, the disease is currently incurable with a high cost of morbidity and mortality. Sotatercept, a new activin receptor IIA-Fc fusion protein, may prove to be a game-changer as a therapeutic agent for the treatment of PAH by regulating the growth factor signaling aberration of PAH.
Methods: It is a narrative review of evidence for the drug Sotatercept for Group 1 PAH from a systematic literature search for clinical trials, mechanism studies, and regulatory data up to 2024. Pivotal clinical trials such as PULSAR, SPECTRA, STELLAR, and ZENITH were evaluated for efficacy, safety, and comparative results.
Results: Sotatercept is a TGF-β family member ligand trap that rebalances activin/BMP signaling to target vascular remodeling. Clinically, striking effects were shown with exercise (40.8 m improvement in STELLAR 6MWD), pulmonary hemodynamics (PVR reduction of 146-240 dyn·s·cm-5 in PULSAR), as well as clinical measures (76% reduction of composite morbidity/mortality through ZENITH). On the background with an acceptable drug safety profile of predominantly hematologic effects, as well as injection site reaction, benefits were achieved. Comparison with analyses implies at least similar, if superior in some dimensions, efficacy of current PAH therapies.
Conclusion: Sotatercept is a new therapeutic option for PAH as the first drug to act on the activin/BMP pathway. With its strong effect on several clinically relevant end points, it is a “fourth pillar” of PAH therapy. Clinical trials will determine its place in the algorithm, ascertain other combinations, and potentially identify its utility for other types of pulmonary hypertension.
The current therapies for PAH target three pathways: the endothelin pathway (endothelin receptor antagonists), the nitric oxide pathway (phosphodiesterase type 5 inhibitors, soluble guanylate cyclase stimulators), and the prostacyclin pathway (prostacyclin analogs, prostacyclin receptor agonists) [1-4]. They have improved survival for PAH patients, yet the disease is incurable, with a survival of around 60% - 70% at 5 years with maximal medical management [5]. This underlines the overwhelming unmet need for novel therapeutic approaches targeting other pathways involved in the pathogenesis of PAH.
More current evidence of the molecular underpinnings of PAH have suggested imbalances of the activin/Bone Morphogenetic Protein (BMP) signaling pathway to be a primary force driving disease progression [6]. Mutations of the gene for the Bone Morphogenetic Protein Receptor type 2 (BMPR2), present in 70% of familial PAH as well as 20% of idiopathic PAH, lead to reduced BMP signaling and susceptibility to remodeling of the pulmonary blood vessels [7]. This led to the development of Sotatercept, a novel activin receptor IIA-Fc fusion protein with the ability to act as a TGF-β superfamily member ligand trap, of which the activins are a part [8].
The current narrative review aims to provide a general overview of Sotatercept for the treatment of Group 1 PAH, the mechanism of action, clinical trial results, safety, and comparative effectiveness with existing therapies. The review will provide areas of research to be conducted in the future as well as studies currently being conducted to provide a clearer understanding of the role of Sotatercept for the treatment of PAH.
Mechanism of action
Sotatercept is a disease-modifying drug under clinical testing for the treatment of PAH that blocks the abnormal growth factor signaling involved in disease pathogenesis. Its mechanism of action is distinct from the approved treatments for PAH as the approved treatments are vasodilation through endothelin, nitric oxide, and prostacyclin pathways [3].
The Transforming Growth Factor-β (TGF-β) family of proteins are homeostatic modulators of vascular homeostasis, growth, and differentiation and are composed of activins as well as Bone Morphogenetic Proteins (BMPs) [4]. Normal pulmonary vasculature is maintained by a qualitative equilibrium of activin-dependent proliferation signaling and BMP-dependent antiproliferation signaling [5]. It is needed for vascular integrity as well as inhibition of undesirable vascular remodeling.
While there is heterogeneity of pathological mechanisms of injury in PAH, there is derangement of the equilibrium of signaling with reduced BMP signaling and increased activin signaling [6]. The majority of PAH results from disease-promoting mutations of the BMPR2 gene that alter mechanisms of signaling, ultimately resulting in decreased BMP signaling, with vascular remodeling potential gaining importance [7]. Circulating activins, as a set of TGF-β superfamily ligands present in increased quantities, are responsible for the derangement with increased pulmonary vascular smooth muscle cell growth, endothelial injury, and vascular remodeling [8].
Sotatercept is a fusion of the extracellular domain of the human activin receptor type IIA (ActRIIA) with the Fc portion of the human immunoglobulin G1 (IgG1) [9]. As a fusion protein, sotatercept is a high affinity ligand trap for several TGF-β superfamily ligands, including activins, Growth Differentiation Factors (GDFs), and several BMPs [6,7,10-16].
At the cellular level, inhibition of activin signaling by Sotatercept triggers a number of therapeutic effects within the pulmonary vasculature:
- Inhibition of proliferation or migration of pulmonary arterial smooth muscle cells
- Restoring endothelial integrity and reversing endothelial apoptosis [12,14,17]
- Preventing harmful extracellular matrix deposition and vascular fibrosis [18]
- Regulation of inflammatory mechanisms involved in vascular remodeling processes [3]
Sotatercept modulates a number of downstream processes that are affected at the molecular level. It blocks activin binding to its receptor and resultant activation of the Smad2/3 pathway with its significance for proliferation and profibrotic action [17]. It decreases instead relative activity of the Smad1/5/8 pathway with its significance for antiproliferation [12]. Smad signaling regulation involved in a number of intracellular processes, reestablishes the relative balance of proliferative vs. antiproliferation signals within pulmonary vasculature.
Sotatercept’s action mechanism is a new mechanism of action relative to current PAH treatments with 3 recognized mechanisms of vasoconstriction:
- They block vasoconstricting and proliferating actions of endothelin-1
- Phosphodiesterase type 5 inhibitors (PDE5 inhibitors) or soluble guanylate cyclase
- Prostacyclin analogues, prostacyclin receptor agonists dilate blood vessels and inhibit platelet aggregation
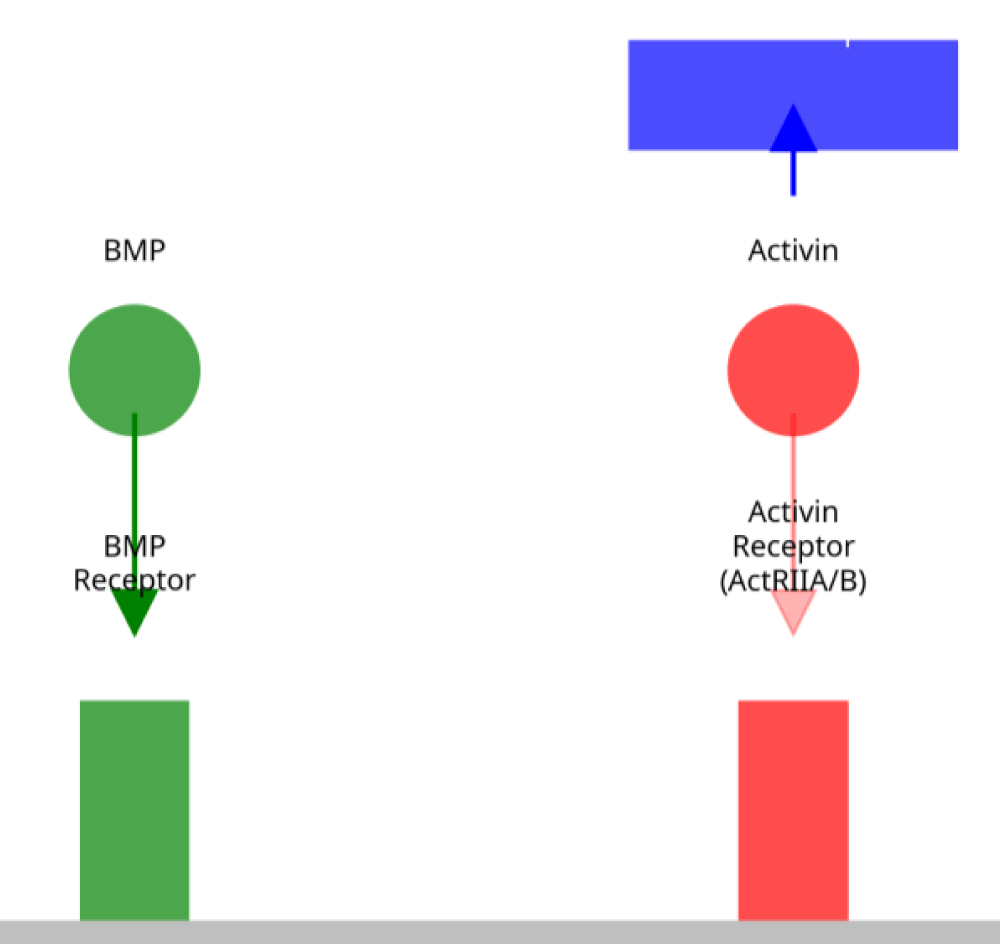
Sotatercept therapy in preclinical models is shown to result in decreased pulmonary vascular remodeling, decreased right ventricular hypertrophy, and improvement of hemodynamics. There are preclinical data that have justified guided investigational studies with sotatercept trial reports under particular conditions with the use of PAH patients Figure 1.
Figure 1: Mechanism of Action of Sotatercept in PAH. Sotatercept (blue) acts as a ligand trap, sequestering excess activin ligands (red) from binding to ActRIIA/B receptors, thereby rebalancing signaling toward BMP-mediated (green) antiproliferative pathways in pulmonary vascular cells.
Summary of clinical trials
The clinical development program for Sotatercept for Pulmonary Arterial Hypertension (PAH) consists of several pivotal trials that have evaluated its efficacy and safety across different patient populations. Below is a summary of the key clinical trials of Sotatercept for PAH with designs, patient populations, interventions, endpoints, and key results.
The PULSAR trial was a double-blind, placebo-controlled, multicenter, randomized phase 2 trial that evaluated the efficacy as well as safety of Sotatercept for patients with PAH [8,9,12,19,20,21]. It was a 24-week trial performed for 106 adults with WHO Functional Class II or III PAH who were receiving stable background therapy.
Patients were allocated 3:3:4 randomly to subcutaneous injection of placebo, 0.3 mg/kg, or 0.7 mg/kg of Sotatercept every 3 weeks alongside their Background PAH management. Change from baseline to week 24 Pulmonary Vascular Resistance (PVR) through right heart catheterization was the main outcome measurement [12].
Sotatercept was linked with statistically significant improvements from baseline versus placebo. Least squares means were approximately -146 dyn·s·cm⁻⁵ for the 0.3 mg/kg dose and -240 dyn·s·cm⁻⁵ for the 0.7 mg/kg dose (both with p < 0.01) [12]. Sotatercept was also significantly beneficial for several secondary endpoints, including placebo-adjusted 6-Minute Walk Distance (6MWD) improvement of 29.4 meters for the 0.3 mg/kg dose, reduction of levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP), and improvement of WHO Functional Class [12].
The SPECTRA trial was a phase 2a open-label trial that specifically evaluated the effect of Sotatercept on right heart function as well as exercise tolerance in patients with PAH [10].
The trial enrolled around 21 patients with WHO Functional Class III PAH. Peak oxygen consumption (VO₂) change through cardiopulmonary exercise testing was the main outcome, while 6MWD and changes in right ventricular function were secondary outcome measures [10].
The STELLAR trial was a multicenter, double-blind, placebo-controlled trial of pivotal design, phase 3, conducted to evaluate the safety as well as efficacy of Sotatercept in a bigger cohort of patients with PAH [9,10].
The double-blind, placebo-controlled, randomized trial allocated around 323 WHO Functional Class II-III PAH patients receiving stable background therapy. The patients were randomly allocated to receive add-on to background therapy with Sotatercept or placebo. 6MWD change from baseline to week 24 was the primary outcome [9].
Sotatercept was statistically superior at the primary end-point, with a median 6MWD gain of 34.4 meters with Sotatercept compared to 1.0 meter with placebo (approximately 40.8 meter difference, p < 0.001) [9]. Further, 8 of the 9 secondary end-points favored Sotatercept as well, with clear evidence of its effectiveness against a number of clinically relevant measures [9]. The STELLAR trial repeated the finding of the prior phase 2 PULSAR trial and confirmed the effectiveness of Sotatercept with a more extensive patient population.
The ZENITH trial was a double-blind, placebo-controlled phase 3 trial that evaluated Sotatercept in high-risk PAH patients [22].
It involved patients with WHO Functional Class III or IV PAH who had a high risk of clinical deterioration. Death, hospitalisation for PAH, or lung transplant was the combined endpoint [22].
With a follow-up of around 10.6 months, a dramatic 76% risk reduction for the primary composite end point was shown with Sotatercept compared with placebo (hazard ratio 0.24, p < 0.0001) [22]. 17.4% of patients with the primary end point were treated with Sotatercept compared with 54.7% of patients receiving a placebo, demonstrating the dramatic clinical utility of Sotatercept with this high-risk patient population [22]. Strong evidence for Sotatercept’s action with hard clinical end points, including mortality and morbidity, was shown with the ZENITH trial.
Sotatercept was studied in a phase 3 trial, the HYPERION trial, for its effectiveness in patients with recently diagnosed high-risk PAH [23].
Particularly, the HYPERION trial was terminated pre-emptively due to loss of equipoise, as a data monitoring committee recommended trial closure due to overwhelming benefit as noted from similar trials [23]. While the final efficacy results have not been published, early trial closure suggests a substantial treatment effect consistent with the results of the other studies being conducted as part of the clinical development program.
CADENCE is a phase 3 trial of sotatercept for the treatment of pulmonary hypertension associated with heart failure with preserved ejection fraction (WHO Group 2 PH).
The trial (NCT04945460 at ClinicalTrials.gov) is testing the safety and efficacy of Sotatercept in a different patient cohort from the previous studies, which were conducted using Group 1 PAH [24]. No full results have been published as of now because the trial continues.
SOTERIA trial is a controlled, open-label trial (NCT04796337, ClinicalTrials.gov) assessing the long-term efficacy and safety of Sotatercept for patients who were previously enrolled in a previous clinical trial [24].
Interim results from the SOTERIA trial have shown that the positive effects of 6MWD as well as NT-proBNP levels noted in the previous studies were, overall, maintained at one year of treatment [25]. Notably, no new safety issues have emerged with prolonged duration of exposure to Sotatercept, with 98.5% of the patients continuing with the treatment at one year, suggesting excellent tolerance [25].
The MOONBEAM trial is a phase 2 trial (ClinicalTrials.gov identifier: NCT05587712) investigating the safety and effectiveness of Sotatercept in pediatric patients with PAH [26]. It is a major expansion of clinical development into the pediatric patient population, where therapies are often restricted. Because the trial is still active, as of yet, results are unavailable.
The baseline data and key efficacy results of these trials are shown in Tables 1 and 2, respectively. Cumulatively, these trials show strong efficacy and safety of Sotatercept in a spectrum of patients with PAH, ranging from those with less severe disease and receiving stable background therapy to high-risk patients. Effects on a spectrum of endpoints, such as exercise tolerance, hemodynamics, biomarkers, and clinical end points, demonstrate the therapeutic utility of Sotatercept to address the underlying pathophysiologic mechanisms of PAH as well as to improve patient outcome.
| Table 1: Baseline Demographics Across Sotatercept Clinical Trials. | ||||
| Characteristic | PULSAR | SPECTRA | STELLAR | ZENITH |
| Study design | Phase 2 RCT | Phase 2a open-label | Phase 3 RCT | Phase 3 RCT |
| Number of patients | 106 | ~21 | ~323 | Not specified |
| WHO functional class | II-III | III | II-III | III-IV (high-risk) |
| Background therapy | Yes | Yes | Yes | Yes |
| Treatment duration | 24 weeks | Not specified | 24 weeks | ~10.6 months (median) |
| Dosing | 0.3 mg/kg or 0.7 mg/kg SC q3w | Not specified | Not specified | Not specified |
| Table 2: Efficacy outcomes across sotatercept clinical trials. | ||||
| Characteristic | PULSAR | SPECTRA | STELLAR | ZENITH |
| Primary endpoint | PVR reduction | Peak VO₂ increase | 6MWD improvement | Composite of death, transplant, or hospitalization |
| 6MWD change | +29.4 m (0.3 mg vs. placebo) | Improved (secondary) | +34.4 m vs. +1.0 m placebo (diff: ~40.8 m, p < 0.001) | Not specified |
| PVR change | -146 and -240 dyn·s·cm⁻⁵ (0.3 and 0.7 mg) | Not specified | Not specified | Not specified |
| NT-proBNP | Reduced | Not specified | Not specified | Not specified |
| WHO-FC | Improved | Not specified | Not specified | Not specified |
| Peak VO₂ | Not specified | +102.7 mL/min (p < 0.01) | Not specified | Not specified |
| RV function | Not specified | Improved | Not specified | Not specified |
| Clinical worsening | Not specified | Not specified | Not specified | 17.4% vs. 54.7% (HR 0.24, p < 0.0001) |
| Secondary endpoints | Not specified | Not specified | 8 of 9 favored Sotatercept | Not specified |
Safety and tolerability
Sotatercept’s safety and tolerability have been examined within a number of clinical trials in patients with PAH. Adverse event profiles are both important for patient selection as well as for clinical decision making. Below is a summary of safety data regarding the pivotal clinical trials of Sotatercept for the treatment of PAH.
During the clinical development program, the safety profile of Sotatercept was consistent with a set of common adverse effects thought to be mechanism-based [8]. The adverse effects most associated with Sotatercept treatment are:
Thrombocytopenia was associated with Sotatercept treatment as well, but it was of a mild to moderate degree [8,9,15]. Thrombocytopenia was noted in nearly 23% of the patients treated with Sotatercept versus 5% receiving placebo in the STELLAR trial.
As a subcutaneous drug, injection site reaction is reported by clinical trials [8]. Injection site reaction consists of mostly erythema, pain, injection site swelling. Injection site reaction occurred in around 16% of the patients administered with Sotatercept in the PULSAR trial [8].
Other side effects as observed in trials are:
- Epistaxis (nosebleeds), most likely resulting from effects on vascular integrity [9]
- Telangiectasia (dilated small blood vessels close to the skin surface) [9]
- Dizziness
- Increased blood pressure, potentially linked with hemoglobin increases and the consequent changes in blood viscosity [9]
Most importantly, there were no unexpected organ toxicities observed under the course of clinical development [9,22]. Its safety profile was consistent with that predicted from its known mechanism of action, with no off-target effects arising that could limit its clinical utility.
Treatment discontinuations due to adverse effects have been low in clinical trials of Sotatercept [8]. During the PULSAR trial, ~9% of the patients treated with Sotatercept had discontinuations due to adverse effects compared with 3% for the placebo arm [8]. Adverse effects leading to discontinuations were most often due to thrombocytopenia and hemoglobin levels that were notably above the level of the protocol [8].
The open-label SOTERIA trial has also shown encouraging long-term tolerance data with approximately the same 98.5% of patients continuing with the treatment up to one year [25]. This extremely high rate of patient retention confirms that the side effects of Sotatercept are generally well tolerated with very little impact on patient continuation of the drug.
The predictability of the most common adverse effects associated with Sotatercept allows for preemptive management and monitoring strategies:
- Hemoglobin is to be monitored routinely, with a reduction in dose or a hold if levels are above set cutoffs [9]
- Platelet levels must be checked from time to time, especially for patients with baseline thrombocytopenia or anticoagulant-treated patients [9]
- Instruct patients on proper injection technique to minimize injection site reactions [8]
Limited data are available regarding the safety of Sotatercept within subpopulations, including pregnant women, pediatric populations, and patients with severe renal or hepatic impairment [27]. Safety data within pediatric PAH patients will be derived from the ongoing MOONBEAM trial [24].
Overall, Sotatercept was generally well tolerated with a favorable safety profile within the clinical trials of PAH patients [09]. Its most common adverse effects are consistent with its mechanism of action and can typically be controlled with monitoring and adjustment of dose. Its lack of unexpected organ toxicities and high rates of completion of the long-term studies are signs that Sotatercept is generally well tolerated within the PAH patient population [25]. With all new therapy, there continues to be a need for ongoing vigilance and post-approval surveillance to better define the long-term safety profile of Sotatercept within routine clinical practice.
Comparative efficacy analysis
To put the therapeutic value of Sotatercept as a treatment for PAH into perspective, its efficacy must be compared with that of established treatments for PAH. This section compares the efficacy of Sotatercept with established treatments for PAH against key clinical end points.
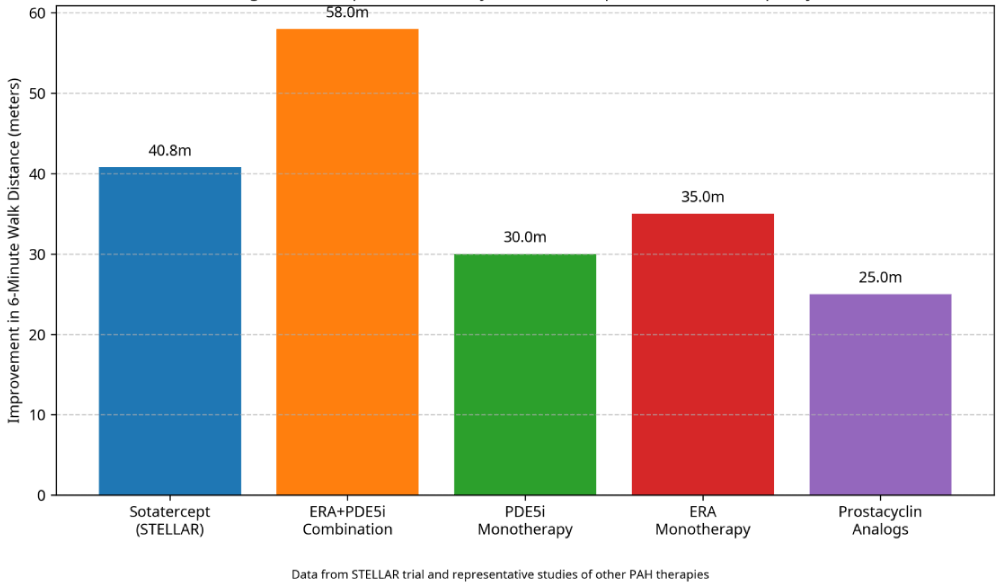
Improvement in exercise capacity, as determined by the 6-minute walk distance (6MWD), is a proven clinical trial outcome for PAH and correlates with functional class and prognosis [1]. As indicated by Figure 2, sotatercept had a clinically relevant improvement of 6MWD with a placebo-adjusted difference of around 40.8 meters (p < 0.001) [9].
Figure 2: Comparative Efficacy of PAH Therapies on Exercise Capacity.
Relative to other treatments for PAH, the 6MWD effect of Sotatercept is substantial:
- ERAs as monotherapy generally result in improvements of around 35 meters [28]
- Phosphodiesterase type 5 inhibitors (PDE5i) have benefits of about 30 meters alone
Prostacyclin analogues had variable efficacy with a mean of about 25 meters.
Most importantly, Sotatercept provided its 40.8-meter add-on over 6MWD to background therapy, often a regimen of combinations of existing PAH therapies [29-31]. This suggests add-on effects with the incorporation of Sotatercept into a regimen, potentially due to its novel mechanism of action against underlying vascular remodeling as distinct from vasodilation.
PVR constitutes a significant hemodynamic indicator of the degree of pulmonary vascular remodeling and obstruction [1]. During the PULSAR trial, Sotatercept was linked with substantial decreases in PVR, with least squares means compared with placebo of approximately -146 dyn·s·cm⁻⁵ for the 0.3 mg/kg dose and -240 dyn·s·cm⁻⁵ for the 0.7 mg/kg dose [8].
Comparative evidence from published meta-analyses of standard PAH therapies provides:
- ERAs decrease PVR by around 220-300 dyn·s·cm
- PDE5i generally cause PVR drops of about 250-300 dyn·s·
Prostacyclin analogs exhibit variable action with declines of 200-400 dyn·s·
The reduction of PVR with Sotatercept also appears of similar degree to that of current treatments, at least with the higher dose [8,32,33]. Inter-study comparisons must, however, be performed with caution due to heterogeneity of trial populations, background treatments, and trial protocols.
N-terminal pro-B-type natriuretic peptide (NT-proBNP), a marker of right ventricular strain, correlates with the severity of PAH as well as with prognosis [1]. Clinical trials have indicated that sotatercept decreases NT-proBNP remarkably, with the PULSAR trial demonstrating median reductions of 51% from baseline for the 0.7 mg/kg dose compared with 9% for placebo [8].
Other studies of treatments for PAH have the following comparative results:
PDE5i generally result in decreases of about 25-35.
The ERA-PDE5i combination results have shown declines of up to 40% - 50% [28,31,34]
The reduction of NT-proBNP with Sotatercept is as much as, if not more than, with traditional treatments of PAH. This is reflective of a significant effect on right ventricular function, one of the most important prognosticators of outcome with PAH.
The effect of therapy upon clinical worsening events and hard endpoints such as hospitalization and death is of utmost significance for PAH [1,9]. Clear evidence of the action of Sotatercept was provided by the ZENITH trial, with a 76% reduction of the composite endpoint of death, transplant for PAH, or hospitalization compared with placebo (hazard ratio 0.24, p < 0.0001) [22].
Comparative trial data of other therapies for PAH show:
- Macitentan was shown by the SERAPHIN trial to reduce risk of morbidity/mortality events by 45% (hazard ratio 0.55)
- GRIPHON trial of selexipag decreased the risk of morbidity/mortality events by 40% (hazard ratio 0.60) [33,34]
- Initial treatment with ambrisentan + tadalafil was shown by the AMBITION trial to reduce the risk of clinical failure events by 50% compared with pooled monotherapy (0.50 hazard ratio) [31].
Data from STELLAR trial and representative studies of other PAH therapies.
Future perspectives and ongoing research
The introduction of a new drug for Pulmonary Arterial Hypertension (PAH), namely Sotatercept, is a milestone development. Like all new treatments, there are several areas of continuing research and issues to be resolved that will define the role of Sotatercept in the clinician’s practice of the future. This chapter outlines the current status of development, ongoing clinical trials, and the promise of the future for Sotatercept for PAH as well as for other diseases.
There are several clinical trials aimed at determining the efficacy and safety of Sotatercept in various patient populations as well as clinical environments:
Several other trials are planned or are under early development to evaluate Sotatercept for:
- PAH associated with diseases of the connective tissue that is less responsive to conventional treatments [1,8,11,12,24,26,22,32,33,35-37].
- PAH resulting from infection with HIV, a distinct etiologic subset [38]
- Combination treatments with inhalation of soluble guanylate cyclase stimulators, exploring synergistic actions [20]
- Group 3 PH, associated with disease of the lung and/or hypoxia, for which current treatments are severely limited [39]
Biomarkers for response to Sotatercept are the topic of ongoing research [12]. Initial evidence show that some of the elements of the TGF-β/BMP pathway at baseline are predictive of response to therapy [09]. Further research into these putative biomarkers could enable more personalized treatments, where clinicians can identify which patients are most likely to respond to Sotatercept therapy.
While clinical trial results so far demonstrate encouraging gains in exercise tolerance, hemodynamics, and short-term clinical measures, the effect of Sotatercept on long-term disease course and survival remains to be fully determined [28]. Findings from the open-label extension trial SOTERIA will be helpful with regards to data regarding response duration and long-term safety, but trials with extended follow-up periods will need to be performed to determine whether Sotatercept changes the disease course or only treats symptoms [25].
Optimisation of Sotatercept in combination therapeutic regimens is another key area of future research [4]. Uncertainty relates to:
- Ideal combinations with existing therapies for PAH [31]
- Drug-drug interactions and composite side effect profiles [31]
Such studies to provide answers to these issues will further establish treatment algorithms and optimize the clinical utility of Sotatercept for clinical practice.
Sotatercept (WINREVAIR) was submitted for regulatory approval and, according to recent reports, was approved as a “fourth pillar” of PAH therapy [11]. With the inclusion of Sotatercept into clinical practice, the following will be needed:
- Formulating final guidelines for patient selection [4]
- Use of monitoring protocols for potential adverse effects
- Training of healthcare providers on the novel mechanism of action and unique considerations for management [20]
- Overcoming potential access barriers including cost and reimbursement issues [40].
The development of sotatercept has also increased awareness of the role of growth factor signaling within the pathogenesis of PAH [139]. More understanding of mechanisms can potentially enable targeting of other therapeutic agents within the TGF-β/BMP pathway or nearby pathways that control vascular remodeling [9]. It may be possible for future research to explore more targeted methods of manipulating particular components of these pathways to achieve maximal benefit with reduced off-target adverse effects.
Sotatercept is a new addition to the therapeutic arsenal for PAH, a new therapeutic approach having a mechanism of action targeting disease pathophysiologic determinants unaddressed by existing treatments. The mechanism of action of sotatercept, clinical data, safety, and relative effectiveness for the management of Group 1 PAH have been elaborated upon within this narrative review.
Sotatercept’s unique mechanism of action as a fusion protein of activin receptor IIA acting as a ligand trap for TGF-β family members makes it stand out from traditional PAH treatments [12]. By rebalancing abnormally regulated growth factor signaling, Sotatercept treats the root vascular remodeling of PAH, as opposed to vasodilation alone [8]. This mechanism of action makes Sotatercept a “fourth pillar” of PAH therapeutics, next to traditional approaches targeting the endothelin, nitric oxide, and prostacyclin pathways [11].
The clinical development of Sotatercept provided robust evidence of efficacy for several clinically relevant endpoints. It was shown to have a substantial benefit on pulmonary vascular resistance as well as 6-minute walking distance in the phase 2 PULSAR trial [8]. It was subsequently confirmed and extended by the pivotal phase 3 STELLAR trial with a clinically significant benefit for exercise capacity [9]. More notably, the ZENITH trial provided strong evidence for the effectiveness of Sotatercept with evidence of a substantial benefit of the composite outcome of death, lung transplant, or hospitalization of high-risk PAH patients [22].
Comparative studies demonstrate that the effectiveness of Sotatercept is similar to, if not superior to, current treatments for PAH for a number of endpoints [9]. Especially noteworthy are the placebo-subtracted improvements observed in exercise performance, hemodynamics, biomarkers, and clinical parameters over background therapy and demonstrate that Sotatercept is a valuable new drug in the therapeutic arsenal against PAH [24].
Despite these promising results, a number of important questions remain to be answered by current and impending research. Among these are the long-term effects of Sotatercept on disease progression and survival, optimal combination therapeutic regimens, and the potential expansion of Sotatercept to pulmonary hypertension of other types beyond Group 1 PAH [9,12,22]. Results from ongoing clinical trials, such as CADENCE and MOONBEAM, will provide valuable insight into these questions.
In summary, Sotatercept has delivered clinically meaningful improvement in the exercise performance, hemodynamics, and clinical status of PAH patients, a therapeutic advancement for the challenging condition. Its novel mechanism of action against the pathologic vascular remodeling can potentially change the disease course rather than merely offering symptomatic relief. As clinical practice with Sotatercept continues to grow and studies progressively clarify its role within the therapeutic arsenal, the new therapy can really have an impact on the outcome of patients with PAH.
Niroshan Ranjan, MD: Conceptualization, manuscript writing, and final review.
Ahmed Ahmed, MD: Data collection and analysis, manuscript writing.
Jordana Woods, MD: Data interpretation, critical review.
Ramaneshwar Selvaraj, MBBS: Literature search, data collection.
Mihir Patel, DO: Manuscript editing, critical review.
Yaman Dalati, DO: Data interpretation, manuscript review.
Vidushan Sabanathan: Assistance with literature review and manuscript preparation.
Thanujan Thangadurai, MD: Data analysis, manuscript editing.
- Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016 Jan 1;37(1):67-119. Available from: https://doi.org/10.1093/eurheartj/ehv317
- Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019 Jan;53(1):1801913. Available from: https://doi.org/10.1183/13993003.01913-2018
- Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019 Jan;53(1):1801887. Available from: https://doi.org/10.1183/13993003.01887-2018
- Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 7;43(38):3618-3731. Available from: https://doi.org/10.1093/eurheartj/ehac237
- Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012 Aug;142(2):448-456. Available from: https://doi.org/10.1378/chest.11-1460
- Morrell NW, Aldred MA, Chung WK, Elliott CG, Nichols WC, Soubrier F, et al. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J. 2019 Jan;53(1):1801899. Available from: https://doi.org/10.1183/13993003.01899-2018
- Soubrier F, Chung WK, Machado R, Grünig E, Aldred MA, Geraci M, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol. 2013 Dec 24;62(25 Suppl):D13–D21. Available from: https://doi.org/10.1016/j.jacc.2013.10.035
- Humbert M, McLaughlin V, Gibbs JS, Gomberg-Maitland M, Hoeper MM, Preston IR, et al. Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med. 2021 Apr 1;384(13):1204–1215. Available from: https://doi.org/10.1056/nejmoa2024277
- Hoeper MM, Badesch DB, Ghofrani HA, Gibbs JSR, Gomberg-Maitland M, McLaughlin VV, et al. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023 Apr 20;388(16):1478-1490. Available from: https://doi.org/10.1056/nejmoa2213558
- Waxman AB, Systrom DM, Manimaran S, de Oliveira Pena J, Lu J, Rischard FP. SPECTRA phase 2b study: impact of sotatercept on exercise tolerance and right ventricular function in pulmonary arterial hypertension. Circ Heart Fail. 2024 May;17(5):e011227. Available from: https://doi.org/10.1161/circheartfailure.123.011227
- U.S. Food and Drug Administration. FDA approves Winrevair (sotatercept-csrk) for adults with pulmonary arterial hypertension. FDA News Release. March 26, 2025. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-winrevair-sotatercept-csrk-adults-pulmonary-arterial-hypertension
- Yung LM, Yang P, Joshi S, Augur ZM, Kim SSJ, Bocobo GA, et al. ACTRIIA-Fc rebalances activin/GDF versus BMP signaling in pulmonary hypertension. Sci Transl Med. 2020;12(543):eaaz5660. Available from: https://doi.org/10.1126/scitranslmed.aaz5660
- Morrell NW, Bloch DB, ten Dijke P, Goumans MJ, Hata A, et al. Targeting BMP signalling in cardiovascular disease and anaemia. Nat Rev Cardiol. 2016;13(2):106-120. Available from: https://doi.org/10.1038/nrcardio.2015.156
- Goumans MJ, Ten Dijke P. TGF-β signaling in control of cardiovascular function. Cold Spring Harb Perspect Biol. 2018;10(2):a022210. Available from: https://doi.org/10.1101/cshperspect.a022210
- Ruckle J, Jacobs M, Kramer W, Pearsall AE, Kumar R, Underwood KW, et al. Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J Bone Miner Res. 2009;24(4):744-752. Available from: https://doi.org/10.1359/jbmr.081208
- Raftopoulos H, Laadem A, Hesketh PJ, et al. Sotatercept (ACE-011) for the treatment of chemotherapy-induced anemia in patients with metastatic breast cancer or advanced or metastatic solid tumors treated with platinum-based chemotherapeutic regimens: results from two phase 2 studies. Support Care Cancer. 2016;24(4):1517-1525. Available from: https://doi.org/10.1007/s00520-015-2962-2
- Goumans MJ, Liu Z, ten Dijke P. TGF-β signaling in vascular biology and dysfunction. Cell Res. 2009;19(1):116-127. Available from: https://doi.org/10.1038/cr.2008.326
- Rol N, Kurakula KB, Happé C, Bogaard HJ, Goumans MJ, et al. TGF-β and BMPR2 signaling in PAH: two black sheep in one family. Int J Mol Sci. 2018;19(9):2585. Available from: https://doi.org/10.3390/ijms19092585
- Galiè N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res. 2004;61(2):227-237. Available from: https://doi.org/10.1016/j.cardiores.2003.11.026
- Klinger JR, Elliott CG, Levine DJ, Bossone E, Duvall L, Fagan K, et al. Therapy for pulmonary arterial hypertension in adults: update of the CHEST guideline and expert panel report. Chest. 2019;155(3):565-586. Available from: https://doi.org/10.1016/j.chest.2018.11.030
- Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889. Available from: https://doi.org/10.1183/13993003.01889-2018
- Channick RN, Frantz RP, Kawut SM. Sotatercept in patients with pulmonary arterial hypertension at high risk of clinical worsening: results from the ZENITH trial. N Engl J Med. 2025;392(13):1201-1212.
- Merck & Co., Inc. Merck announces early termination of HYPERION trial of sotatercept in pulmonary arterial hypertension due to overwhelming efficacy. Press Release. January 15, 2025. Available from: https://www.merck.com/news/merck-announces-decision-to-stop-phase-3-hyperion-trial-evaluating-winrevair-sotatercept-csrk-early-and-move-to-final-analysis/
- ClinicalTrials.gov. A study of sotatercept for the treatment of pulmonary hypertension associated with heart failure with preserved ejection fraction (CADENCE). NCT04945460. Available from: https://clinicaltrials.gov/study/NCT04945460. Accessed April 10, 2025.
- Benza RL, Farber HW, Frost A. SOTERIA: one-year interim analysis of an open-label extension study of sotatercept for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2024;209:A3809.
- ClinicalTrials.gov. A study to evaluate the safety, tolerability, and efficacy of sotatercept in pediatric participants with pulmonary arterial hypertension (MOONBEAM). NCT05587712. Available from: https://clinicaltrials.gov/study/NCT05587712. Accessed April 10, 2025.
- U.S. Food and Drug Administration. Winrevair (sotatercept-csrk) prescribing information. March 2025.
- Galiè N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117(23):3010-3019. Available from: https://doi.org/10.1161/circulationaha.107.742510
- Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148-2157. Available from: https://doi.org/10.1056/nejmoa050010
- Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334(5):296-301. Available from: https://doi.org/10.1056/nejm199602013340504
- Galiè N, Barberà JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373(9):834-844. Available from: https://doi.org/10.1056/nejmoa1413687
- Petrović M, Locatelli I, Bernjak A. A comparative study of the efficacy of endothelin receptor antagonists and phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension: a meta-analysis of randomized controlled trials. Respir Med. 2020;171:106101. Available from: https://doi.org/10.1016/j.rmed.2020.106101
- Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522-2533. Available from: https://doi.org/10.1056/nejmoa1503184
- Pulido T, Adzerikho I, Channick RN, Delcroix M, Galiè N, Ghofrani HA, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369(9):809-818. Available from: https://doi.org/10.1056/nejmoa1213917
- Vachiéry JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53(1):1801897. Available from: https://doi.org/10.1183/13993003.01897-2018
- Rosenzweig EB, Abman SH, Adatia I, Beghetti M, Bonnet D, Haworth S, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53(1):1801916. Available from: https://doi.org/10.1183/13993003.01916-2018
- Chung L, Farber HW, Benza R, Miller DP, Parsons L, Hassoun PM, et al. Unique predictors of mortality in patients with pulmonary arterial hypertension associated with systemic sclerosis in the REVEAL registry. Chest. 2014;146(6):1494-1504. Available from: https://doi.org/10.1378/chest.13-3014
- Sitbon O, Gressin V, Speich R, Macdonald PS, Opravil M, Cooper DA, et al. Bosentan for the treatment of human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;170(11):1212-1217. Available from: https://doi.org/10.1164/rccm.200404-445oc
- Nathan SD, Barbera JA, Gaine SP, Harari S, Martinez FJ, Olschewski H, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53(1):1801914. Available from: https://doi.org/10.1183/13993003.01914-2018
- Sikirica M, Iorga SR, Bancroft T, Potash J. The economic burden of pulmonary arterial hypertension (PAH) in the US on payers and patients. BMC Health Serv Res. 2014;14:676. Available from: https://doi.org/10.1186/s12913-014-0676-0