More Information
Submitted: March 14, 2024 | Approved: April 19, 2024 | Published: April 22, 2024
How to cite this article: Villamizar JP, Khalid L, Faraj EN, Harrington TJ, De La Zerda DJ. Macitentan in Adults with Sickle Cell Disease and Pulmonary Hypertension: A Proof-of-Concept Study. J Pulmonol Respir Res. 2024; 8: 029-034.
DOI: 10.29328/journal.jprr.1001055
Copyright License: © 2024 Villamizar JP, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Pulmonary vascular disease; Endothelin receptor antagonist; Functional capacity
Macitentan in Adults with Sickle Cell Disease and Pulmonary Hypertension: A Proof-of-Concept Study
Jeany P Villamizar, Laiqua Khalid, Emilia N Faraj, Thomas J Harrington and David J De La Zerda*
Division of Pulmonary, Critical Care Medicine and Sleep Department of Medicine, University of Miami Miller School of Medicine, USA
*Address for Correspondence: David J De La Zerda, MD, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of Miami 1951 NW 7th Ave, Suite 2278, Miami, Florida, 33136, USA, Email: [email protected]
Sickle cell disease (SCD) occurs due to a point mutation at the sixth position of the beta-globin chain of the hemoglobin resulting in the substitution of valine for glutamic acid and synthesis of a structurally abnormal hemoglobin (HbS); this change allows HbS to polymerize when deoxygenated and is responsible for the clinical presentation of SCD including vasoocclusive events and hemolysis [1].
Pulmonary hypertension (PH) is present in approximately 30% of patients with SCD and is associated with a 37% higher mortality in patients with SCD without PH [2]. There is a relationship between hemolysis markers and PH whereby plasma hemoglobin (Hb) scavenges nitric oxide (NO) leading to acute and chronic pulmonary vasoconstriction [3]. In addition, this results in the upregulation of adhesion molecules which leads to the expression of endothelin-1, a potent vasoconstrictor [3].
Clinically, PH is classified into five groups by the World Health Organization (WHO). PH associated with chronic hemolytic anemia including SCD is included in group 5 [4]. Typical findings in SCD-associated PH are elevated cardiac output (CO), left heart disease (LHD), thromboembolic disease, altered blood viscosity, and endothelial dysfunction, with the latter mainly due to NO depletion by free hemoglobin [5].
Clinical trials of pulmonary vasodilator medications in PH of SCD have been problematic. Three randomized placebo-controlled trials have been undertaken previously. Two compared treatment with bosentan to placebo in SCD patients with right heart catheterization (RHC)-defined elevated pulmonary vascular resistance (PVR) with a normal pulmonary capillary wedge pressure (PCWP) (the ASSET-1 trial) or pulmonary venous hypertension (PVH) with a PVR ≥ 100 dynes.sec/cm5 (the ASSET-2 trial) [6].
After the randomization of only 14 subjects in ASSET-1 and 12 patients in ASSET-2, the trials were prematurely terminated due to slow patient enrollment. Although very few patients were enrolled, there were no apparent toxicity issues. The third trial, Walk-PHaSST (Pulmonary Hypertension and Sickle Cell Disease with Sildenafil Therapy) [7], compared the safety and efficacy of sildenafil to placebo in SCD patients with a TRV ≥ 2·7m/s. After 74 (of a targeted 132) subjects were enrolled, the study was prematurely discontinued due to an increase in serious adverse events in the sildenafil group, primarily hospitalization for pain.
Currently, there is no specific treatment for PH in SCD. Clinical practice guidelines published by the American Thoracic Society for the management of PH in SCD suggest a trial with a prostacyclin agonist or an endothelin receptor antagonist (ERA) in patients with SCD who have a right heart catheterization (RHC)-confirmed elevation of pulmonary vascular resistance (PVR), normal pulmonary artery wedge pressure (PAWP), and have symptoms attributable to PH [8]. However, the recommendations are weak due to the low level of evidence [8].
Macitentan is a dual endothelin receptor antagonist (ERA) that has been approved for the long-term treatment of PH. Supportive evidence for this has been provided by studies showing improved pulmonary hemodynamics parameters after six months of treatment irrespective of WHO functional class, with a reduction in morbidity and mortality [9]. Those trials did not include patients with SCD-related PH. Our prospective study aimed to assess the efficacy and safety of a 4-month treatment with macitentan in SCD patients with PH. Relevant outcomes were pulmonary and systemic hemodynamics, exercise capacity, functional class, and quality of life.
Objectives
We aim to describe the effect of endothelin receptor antagonist (macitentan) in patients with pre-capillary pulmonary hypertension due to underlying SCD.
Study design
Approval for this study was obtained from the Institutional Review Board of the University of Miami, number 20170114. A prospective, descriptive study was done to determine the safety and efficacy of macitentan in patients with SCD and PH followed at the University of Miami Hospital and Jackson Memorial Hospital from March 1, 2018, to February 28, 2020. Subjects were screened with echocardiographic determination of tricuspid regurgitant jet velocity (TRV) > 3 m/sec or right ventricular systolic pressure (RVSP) > 40 mmHg. Before initiating therapy and follow-up at weeks 4, 8, 12, and 16 were completed, data collected included echocardiographic imaging, hemodynamics obtained at right heart catheterization, 6-min walk test (6MWT), laboratory analysis, physical examination, Borg dyspnea scores, World Health Organization (WHO) Functional Class (FC), and Short Form 36 Health Survey Questionnaire (SF-36). Baseline laboratory analysis included complete blood count, chemistries, lactate dehydrogenase (LDH), NT-proBNP (N-terminal pro-brain natriuretic peptide), and pregnancy tests for female patients. Additionally, right heart catheterization was repeated at week 16 to determine the change in pulmonary hemodynamics.
Study patients
We screened 13 patients and recruited five. All five patients were adults. Their baseline demographic, echocardiographic, and blood test data are shown in Table 1. Their hemoglobinopathy type was determined by Hb electrophoresis. All were in a stable phase of their disease at the time of evaluation. They provided written informed consent. They were selected based on a suspicion of PH by echocardiography within the last six months and WHO FC Class II or III symptoms. The diagnosis of PH was established by RHC; inclusion criteria were a mean pulmonary artery mean pressure mPAP) > 25 mmHg, pulmonary arterial wedge pressure (PAWP) < 15 mmHg, and PVR > 160 dynes-sec/cm5 or 2 wood units.
Exclusion criteria
Criteria for exclusion from this study included: 1) pregnancy; 2) stroke within six weeks; 3) pulmonary embolism (PE) within the last three months; 4) a positive Human Immunodeficiency Virus (HIV) test; 5) serum alanine aminotransferase (ALT) level greater than or equal to 2 x upper normal limit; 6) positive Hepatitis B surface antigen or Hepatitis C antibody; 7) serum creatinine greater than or equal to 2·5 mg/dL (or calculated creatinine clearance less than or equal to 30mL/min); 8) hospitalization within the prior four weeks for a vasoocclusive crisis or acute chest syndrome; 9) evidence of left ventricular dysfunction (left ventricular ejection fraction < 50% or significant diastolic dysfunction); 10) significant ischemic, valvular or constrictive heart disease.
Follow-up
We performed prospective follow-up evaluations with scheduled appointments at weeks 4,8, 12, and 16 with an assessment of laboratory analysis, 6MWT, Borg Dyspnea scale, SF-36 questionnaire, and functional class.
The intensity of adverse events was graded on a mild, moderate, and severe three-point scale. Mild may be noticeable to the subject but does not influence daily activities and usually does not require intervention. Moderate may make the subject uncomfortable, the performance of daily activity may be influenced, and intervention may be needed. Severe may cause noticeable discomfort and usually interfere with daily activities, and the subject may not be able to continue in the study, and treatment or intervention is usually needed.
Statistical analyses
All statistical analyses were performed using SPSS v26·0 (SPSS, Inc., Chicago, IL). Continuous variables were reported as mean ± SEM or percentage where appropriate. Data were analyzed as appropriate by student t-test. Statistical significance was assumed at p <0.05.
Baseline characteristics
The baseline characteristics of all five patients are shown in Table 1. Baseline pulmonary hemodynamics obtained by right heart catheterization and systemic hemodynamics are shown in Table 2. The mean (± SEM) right heart catheterization values were mean pulmonary artery pressure (MPAP) 32 ± 8 mmHg, right atrial pressure (RAP) 9 ± 4 mmHg, pulmonary vascular resistance (PVR) 257 dynes-sec/cm5 and CI 3.7 ± 0.39 l/m2. The sickle cell phenotype was hemoglobin SS disease (HbSS) in four patients, and sickle hemoglobin C disease (HbSC) in one. Sixty percent of the patients were male, and forty percent were female. The average age was 42.6 yr. Four patients were vasodilatory therapy naïve. Patient 3 was receiving sildenafil before starting the study. Four patients were receiving hydroxyurea as SCD-specific therapy. The mean baseline serum creatinine was 1.01 mg/dl (± 0.29). The mean RVSP by echocardiography was 51 mmHg (± 6).
| Table 1: Characteristics of patients at baseline. | |||||
| Patient N° | |||||
| Characteristics | 1 | 2 | 3 | 4 | 5 |
| Gender | Male | Female | Male | Female | Male |
| Age (years) | 38 | 42 | 50 | 54 | 29 |
| BMI (kg/m2) | 22.2 | 30.3 | 15.7 | 20.1 | 19.4 |
| SCD type | HbSS | HbSC | HbSS | HbSS | HbSS |
| RVSP (mmHg) | 30 | 48.2 | 54 | 59 | 64 |
| 6 MWT (m) | 362.2 | 271.2 | 173.7 | 304.7 | 1210 |
| Functional class | II | II | III | II | III |
| White cell count (103/mm3) | 6.9 | 11.3 | 6.7 | 9.9 | 5.6 |
| Platelet count (103/mm3) | 319 | 292 | 215 | 462 | 649 |
| Blood urea nitrogen (mg/dl) | 9 | 4 | 46 | 25 | 5 |
| Creatinine (mg/dl) | 0.86 | 0.51 | 0.94 | 2.16 | 0.6 |
| Bilirubin (mg/dl) Total Direct | 2.3 | 3.1 | 2.1 | 0.8 | 3.8 |
| 0.4 | 1.2 | 1.1 | 0.2 | 0.5 | |
| Alanine aminotransferase (U/liter) | 25 | 68 | 121 | 36 | 21 |
| Aspartate aminotransferase (U/liter) | 16 | 29 | 75 | 21 | 18 |
| Lactate dehydrogenase (U/liter) | 494 | 1085 | 744 | 624 | 342 |
| NT-proBNP (pg/ml) | 38 | 132.2 | 646.7 | 403.9 | 10.3 |
| Hemoglobin (mg/dl) | 11 | 7.4 | 9.8 | 6.1 | 11 |
| Concomitant therapy | Hydroxyurea | Hydroxyurea, furosemide | Deferasirox, Sildenafil, bumetanide | Epoetin | Hydroxyurea, apixaban |
| BMI: Body Mass Index; TRV: Tricuspid Regurgitant Velocity; 6 MWT: Six-Minute Walk Test; NT-proBNP: N-Terminal Prohormone of Brain Natriuretic Peptide; SCD: Sickle Cell Disease: HbSS: Homozygous Sickle Cell Disease; HbSC: Sickle Hemoglobin C Disease | |||||
| Table 2: Baseline hemodynamic parameters. | ||||||||
| Patient | MPAP (mmHg) | sPAP (mmHg) | RAP (mmHg) | PVR (dynes-sec/cm5) | CO (l/min) | CI (l/m2) | PCWP (mmHg) | SVR (dynes-sec/cm5) |
| 1 | 26 | 35 | 7 | 145 | 6.59 | 3.61 | 14 | 1080.4 |
| 2 | 38 | 56 | 15 | 304 | 5.92 | 3.2 | 17 | 1013.5 |
| 3 | 40 | 67 | 11 | 262 | 7.59 | 3.44 | 15 | 853 |
| 4 | 28 | 41 | 7 | 170 | 6.57 | 4.02 | 12 | 1156.8 |
| 5 | 28 | 49 | 5 | 407 | 4.12 | 4.3 | 11 | 1629 |
| sPAP: systolic Pulmonary Artery Pressure; MPAP: Mean Pulmonary Artery Pressure; PVR: Pulmonary Vascular Resistance; CO: Cardiac Output; CI: Cardiac Index; PCWP: Pulmonary Capillary Wedge Pressure; SVR: Systemic Vascular Resistance | ||||||||
Effects of macitentan
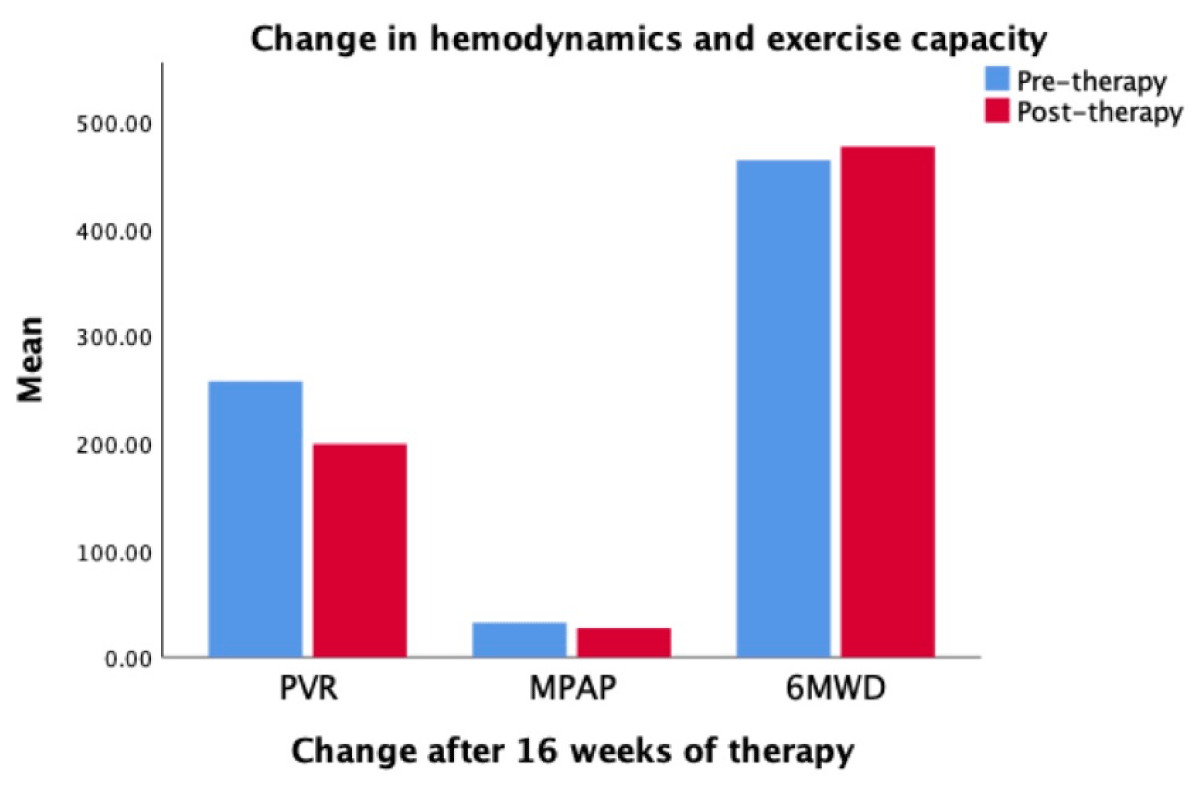
These are shown in Table 3 and Figure 1. Of all parameters, only PVR and 6-min walk distance changed significantly. For the group, MPAP decreased by 15.6%, PVR by 22.5% and RAP by 25.5%. The 6-minute walk distance increased over sixteen weeks except in Patient 4 who had a 3% decrease. The mean walk distance increased in the total distance, from 464 ± 158 meters to 477 ± 190 meters (p =·123). The effect of macitentan on functional class classification and Borg Dyspnea scale was variable, details are in Table 3.
| Table 3: Effect of macitentan on hemodynamics, exercise capacity, and functional class. | ||||||||||||
| MPAP (mmHg) | RAP (mmHg) | PVR (dynes-sec/cm5) | CI (l/m2) | 6 MWD (m) | Functional Class | |||||||
| N° | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| 1 | 26 | 14 | 7 | 1 | 145 | 75 | 3.61 | 3.49 | 362.6 | 393.1 | II | II |
| 2 | 38 | 31 | 15 | 13 | 304 | 158 | 3.2 | 3.41 | 271.2 | 278.9 | II | II |
| 3 | 40 | 27 | 11 | 13 | 262 | 219 | 3.44 | 4.18 | 173.7 | 188.9 | III | II |
| 4 | 28 | 22 | 7 | 22 | 170 | 153 | 4.02 | 2.55 | 304.7 | 295.7 | II | II |
| 5 | 28 | 41 | 5 | 8 | 407 | 391 | 4.3 | 4.3 | 1210 | 1230 | III | III |
| Mean (SEM) | 32 (± 2.8) | 27 (± 4.5) | 9 (± 4) | 11 (± 7.7) | 257 (± 47) | 199 (± 53) | 3.7 (± 0.19) | 3.5 (± 0.31) | 464 (± 188) | 477 (± 190) | ||
| p - value | .347 | .534 | .072 | .744 | .123 | .272 | ||||||
| MPAP: Mean Pulmonary Artery Pressure; RAP: Right Atrial Pressure; PVR: Pulmonary Vascular Resistance; CI: Cardiac Index; 6MWD: Six-Minute Walk Distance; FC: Functional Class; SEM: Standard Error of the Mean; N°: Patient Number | ||||||||||||
Figure 1: Hemodynamic changes measured by right heart catheterizations after completing 16 weeks of treatment. Pulmonary Vascular Resistance (PVR) decreased by 22.5%, mean pulmonary after pressure (MPAP) decreased by 15.6%, and exercise capacity measured by six-minute walk distance (6MWD) increased by 2.8%.
Furthermore, we found improvement in the median NT-proBNP by 71.3%, with specific improvement in patients 1, 3, and 4. Serum LDH level decreased by 7.1%. There was no significant increase in aspartate aminotransferase from baseline 59.25 U/L (± 18.63) to 61.25 U/L (± 9.6) and alanine aminotransferase from 32.5 U/L (± 10.5) to 28 U/L ( 8.6).
Adverse events while on therapy with 10 mg of macitentan occurred in all five patients (Table 4). In four patients, the adverse events were mild to moderate and did not lead to study drug discontinuation. Therapy was discontinued in one patient due to worsening anemia (serious event), although it was uncertain whether this was attributable to the study dug.
| Table 4: Adverse events per patient. | |
| Patient N° | Adverse event |
| 1 | Headache and skin rash |
| 2 | Lower extremity edema, skin rash, dyspnea and nasopharyngitis |
| 3 | Headache, dyspnea, and epistaxis |
| 4 | Lower extremity edema, dyspnea, nasopharyngitis, and decreased Hemoglobin |
| 5 | Dyspnea |
| Adverse event from week 0 to week 16 of follow-up | |
Disease progression
During the 16-week study period; during this period, none of the patients required hospitalization for PAH or the addition of a prostanoid (Table 5). No patient underwent lung transplantation and there were no deaths. In the one patient who was already on chronic oxygen therapy when entering the study, oxygen requirements did not increase.
| Table 5: Adverse events on therapy and disease progression. | |||
| Adverse events | Disease progression | ||
| Number of patients (%) |
Number of patients | ||
| Headache | 2 (40%) | Need for lung transplantation | 0 |
| Nasopharyngitis | 2 (40%) | ||
| Decrease Hb | 1 (20% | Hospitalization for PH | 0 |
| Epistaxis | 1 (20%) | Initiation of IV/SC prostanoids | 0 |
| Dyspnea | 3 (60%) | ||
| Lower extremities edema | 2 (40%) | Chronic oxygen therapy | 1(20%) |
| Skin rash | 2 (40%) | Death | 0 |
| PH: Pulmonary Hypertension; IV: Intravenous; SC: Subcutaneous; SOB: Shortness of Breath | |||
In this prospective study, 4-month monotherapy with macitentan, an ERA receptor blocker, significantly improved pulmonary hemodynamics and exercise capacity in patients with SCD-related pulmonary arterial hypertension. We found that macitentan was safe and well tolerated. Four patients developed mild to moderate adverse events that did not lead to withdrawal of macitentan. Only one patient had a severe adverse event that required discontinuation of therapy due to worsening anemia. The pattern of adverse events in our patients with SCD was similar to what was reported in the SERAPHIN study involving patients with PH due to a variety of other causes [10].
Previous studies have demonstrated that patients with SCD in steady state have elevated levels of ET-1 when compared to healthy individuals [11], although it remains unspecified what percentage of these patients have PH. ET-1 induces vasoconstriction, inflammation, fibrosis, and cellular proliferation when it binds to its receptors ET-A and ET-B. Blood levels of ET-1 are elevated in patients with SCD suffering from a vasoocclusive crisis and PH [11]. Several cytokines can activate the Gardos channel in human erythrocytes; when ET-1 binds to ET-B, it activates the membrane Gardos channels, which causes dehydration of the RBCs and increases hemolysis [12]. Our data suggest that macitentan may benefit from reducing vasoocclusive crisis, considering that none of the patients had events during the study period.
The prevalence of PH in hemolytic anemia is variable; in SCD (HbSS), 30% - 40% of patients have evidence of elevated pulmonary artery pressures, being PH recognized as a major source of morbidity and mortality [13]. In the PUSH study, elevated tricuspid regurgitant jet velocity values of ≥ 2.7 m/second, or more than two standard deviations (SD) above the mean, were significantly associated with death, observed in 20% of patients who died and 4.6% of those who survived [14]. However, other studies found the cutoff higher tricuspid regurgitant jet velocity > 3 m/second [15] in concordance with our study cutoff for screening.
The results of our prospective study are similar to those of a retrospective case series that showed the safety and efficacy of ET receptor blockade in SCD-associated PH, with a significant improvement in the 6-minute walk distance and a decrease in MPAP in the three patients that underwent repeat RHC [14]. While those authors observed a trend toward decreasing NT-proBNP and LDH serum levels during macitentan therapy, we failed to show consistent changes in these parameters.
Although our study population was small, the findings are meaningful as improvements in pulmonary hemodynamics and exercise capacity were seen in all patients. Changes in the 6-minute walk test are considered independent predictors of mortality [16]. Another strength of our study was the use of repeat RHC in all patients for a reliable assessment of pulmonary hemodynamics. Similarly, one of our patients (20%) was on dual PAH therapy with phosphodiesterase five inhibitors compared to 43% of their population.
There are no previous data on the use of macitentan in SCD-associated PH. The study by Minniti, et al. [17] used either bosentan or ambrisentan. The ASSET trial, the largest trial to the date of ERA use in SCD-associated PH, used bosentan but had to be stopped early due to withdrawal of support by the sponsor. Compared to bosentan and ambrisentan, macitentan exhibits higher antagonistic potency and longer duration of action, due to the longer half-life of its active metabolite and slower receptor dissociation kinetics [18].
Our study was not randomized and placebo-controlled. However, improved pulmonary hemodynamics and exercise capacity were consistently seen in all patients. In addition, macitentan had a good safety profile in this population. Our observation could serve as the basis for a larger placebo-controlled study.
Author contribution
ENF had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. JPV, LK, ENF, TJH, and DJD contributed substantially to the study design, data analysis, and interpretation, and the writing of the manuscript.
- Shah F, Dwivedi M. Pathophysiology and recent therapeutic insights of sickle cell disease. Ann Hematol. 2020 May;99(5):925-935. doi: 10.1007/s00277-020-03977-9. Epub 2020 Mar 10. PMID: 32157419.
- Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004 Feb 26;350(9):886-95. doi: 10.1056/NEJMoa035477. PMID: 14985486.
- Rybicki AC, Benjamin LJ. Increased levels of endothelin-1 in plasma of sickle cell anemia patients. Blood. 1998 Oct 1;92(7):2594-6. PMID: 9746804.
- Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano-Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke-Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S; ESC/ERS Scientific Document Group. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-3731. doi: 10.1093/eurheartj/ehac237. Erratum in: Eur Heart J. 2023 Apr 17;44(15):1312. PMID: 36017548.
- Fonseca GH, Souza R, Salemi VM, Jardim CV, Gualandro SF. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J. 2012; 39(1):112-118. doi:10.1183/09031936.00134410
- Barst RJ, Mubarak KK, Machado RF, Ataga KI, Benza RL, Castro O, Naeije R, Sood N, Swerdlow PS, Hildesheim M, Gladwin MT; ASSET study group*. Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies. Br J Haematol. 2010 May;149(3):426-35. doi: 10.1111/j.1365-2141.2010.08097.x. Epub 2010 Feb 17. PMID: 20175775; PMCID: PMC2914575.
- Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, Gordeuk VR, Gibbs JS, Little JA, Schraufnagel DE, Krishnamurti L, Girgis RE, Morris CR, Rosenzweig EB, Badesch DB, Lanzkron S, Onyekwere O, Castro OL, Sachdev V, Waclawiw MA, Woolson R, Goldsmith JC, Gladwin MT; walk-PHaSST Investigators and Patients. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011 Jul 28;118(4):855-64. doi: 10.1182/blood-2010-09-306167. Epub 2011 Apr 28. PMID: 21527519; PMCID: PMC3148167.
- Klings ES, Machado RF, Barst RJ, Morris CR, Mubarak KK, Gordeuk VR, Kato GJ, Ataga KI, Gibbs JS, Castro O, Rosenzweig EB, Sood N, Hsu L, Wilson KC, Telen MJ, Decastro LM, Krishnamurti L, Steinberg MH, Badesch DB, Gladwin MT; American Thoracic Society Ad Hoc Committee on Pulmonary Hypertension of Sickle Cell Disease. An official American Thoracic Society clinical practice guideline: diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease. Am J Respir Crit Care Med. 2014 Mar 15;189(6):727-40. doi: 10.1164/rccm.201401-0065ST. PMID: 24628312; PMCID: PMC3983842.
- Pulido T, Adzerikho I, Channick RN, Delcroix M, Galiè N, Ghofrani HA, Jansa P, Jing ZC, Le Brun FO, Mehta S, Mittelholzer CM, Perchenet L, Sastry BK, Sitbon O, Souza R, Torbicki A, Zeng X, Rubin LJ, Simonneau G; SERAPHIN Investigators. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013 Aug 29;369(9):809-18. doi: 10.1056/NEJMoa1213917. PMID: 23984728.
- Galiè N, Jansa P, Pulido T, Channick RN, Delcroix M, Ghofrani HA, Le Brun FO, Mehta S, Perchenet L, Rubin LJ, Sastry BKS, Simonneau G, Sitbon O, Souza R, Torbicki A. SERAPHIN haemodynamic substudy: the effect of the dual endothelin receptor antagonist macitentan on haemodynamic parameters and NT-proBNP levels and their association with disease progression in patients with pulmonary arterial hypertension. Eur Heart J. 2017 Apr 14;38(15):1147-1155. doi: 10.1093/eurheartj/ehx025. PMID: 28329315; PMCID: PMC5400052.
- Graido-Gonzalez E, Doherty JC, Bergreen EW, Organ G, Telfer M, McMillen MA. Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood. 1998 Oct 1;92(7):2551-5. PMID: 9746797.
- Rivera A, Jarolim P, Brugnara C. Modulation of Gardos channel activity by cytokines in sickle erythrocytes. Blood. 2002 Jan 1;99(1):357-603. doi: 10.1182/blood.v99.1.357. PMID: 11756192.
- Al-Qadi M, LeVarge B, Ford HJ. Epidemiology, Pathogenesis, and Clinical Approach in Group 5 Pulmonary Hypertension. Front Med (Lausanne). 2021 Mar 25;7:616720. doi: 10.3389/fmed.2020.616720. PMID: 33842491; PMCID: PMC8026868.
- Nouraie M, Darbari DS, Rana S, Minniti CP, Castro OL, Luchtman-Jones L, Sable C, Dham N, Kato GJ, Gladwin MT, Ensing G, Arteta M, Campbell A, Taylor JG 6th, Nekhai S, Gordeuk VR. Tricuspid regurgitation velocity and other biomarkers of mortality in children, adolescents and young adults with sickle cell disease in the United States: The PUSH study. Am J Hematol. 2020 Jul;95(7):766-774. doi: 10.1002/ajh.25799. Epub 2020 Apr 21. PMID: 32243618; PMCID: PMC7735042.
- Upadhya B, Stacey RB, Ntim W, Knovich MA, Pu M. Echocardiography-derived tricuspid regurgitant jet velocity is an important marker for the progression of sickle-cell disease. Acta Haematol. 2014;132(2):152-8. doi: 10.1159/000357393. PMID: 24577318.
- Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, Nakanishi N, Miyatake K. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000 Feb;161(2 Pt 1):487-92. doi: 10.1164/ajrccm.161.2.9906015. PMID: 10673190.
- Minniti CP, Machado RF, Coles WA, Sachdev V, Gladwin MT, Kato GJ. Endothelin receptor antagonists for pulmonary hypertension in adult patients with sickle cell disease. Br J Haematol. 2009 Dec;147(5):737-43. doi: 10.1111/j.1365-2141.2009.07906.x. Epub 2009 Sep 22. PMID: 19775299; PMCID: PMC3225273.
- Gatfield J, Mueller Grandjean C, Sasse T, Clozel M, Nayler O. Slow receptor dissociation kinetics differentiate macitentan from other endothelin receptor antagonists in pulmonary arterial smooth muscle cells. PLoS One. 2012;7(10):e47662. doi: 10.1371/journal.pone.0047662. Epub 2012 Oct 15. PMID: 23077657; PMCID: PMC3471877.