More Information
Submitted: January 27, 2024 | Approved: March 18, 2024 | Published: March 19, 2024
How to cite this article: Swanevelder C, Prasad L, Chen KYY, Zeng I, Corna N, et al. Intradermal and Subcutaneous Lignocaine for Arterial Blood Gas Sampling: A Randomized Controlled Trial. J Pulmonol Respir Res. 2024; 8: 023-028.
DOI: 10.29328/journal.jprr.1001054
Copyright License: © 2024 Swanevelder C, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Arterial blood gas; Intradermal lignocaine; Subcutaneous lignocaine; Local anesthesia; Oxygen clinic
Intradermal and Subcutaneous Lignocaine for Arterial Blood Gas Sampling: A Randomized Controlled Trial
Charlene Swanevelder1, Lila Prasad1, Kevin YY Chen2, Irene Zeng3, Nicola Corna4, Anh Nguyen5 and Conroy Wong6*
1Clinical Nurse Specialist, Department of Respiratory Medicine, Middlemore Hospital, Te Whatu Ora Counties Manukau, Auckland, New Zealand
2Respiratory Registrar, Department of Respiratory Medicine, Middlemore Hospital, Te Whatu Ora Counties Manukau, Auckland, New Zealand
3Middlemore Hospital, Te Whatu Ora, Counties Manukau, Auckland, New Zealand
4Respiratory Nurse Practitioner, Department of Respiratory Medicine, Middlemore Hospital, Te Whatu Ora Counties Manukau, Auckland, New Zealand
5Pharmacist, Department of Pharmacy, Middlemore Hospital, Te Whatu Ora Counties Manukau, Auckland, New Zealand
6Respiratory Physician, Department of Respiratory Medicine, Middlemore Hospital, Te Whatu Ora, and University of Auckland, Auckland, New Zealand
*Address for Correspondence: Conroy Wong, Associate Professor, Department of Respiratory Medicine, Middlemore Hospital, Te Whatu Ora Counties Manukau, Auckland, New Zealand, Email: [email protected]
Introduction: The use of local anesthesia (LA) prior to arterial blood gas sampling is recommended but is not widely used. We tested the hypothesis that intradermal administration of local anesthesia would be as effective as subcutaneous administration in reducing pain from arterial blood gas sampling.
Aims: The primary aim of this study was to evaluate the effect of intradermal and subcutaneous lignocaine on patient-perceived pain during arterial blood gas sampling. The secondary aims were to evaluate if different routes of LA administration had an impact on the difficulty and complications of ABG sampling.
Methods: We undertook a randomized, single-blind, placebo-controlled trial in New Zealand. We enrolled patients attending a nurse-led outpatient oxygen clinic who were 18 to 90 years of age and who had an oxygen saturation of 93% or less at rest. Patients were randomly assigned to receive intradermal 1% lignocaine, subcutaneous 1% lignocaine, or subcutaneous normal saline. Patients and nurse assessors were blinded to the treatment allocation. The primary endpoint was a patient-assessed pain score using a graphic rating scale (0-10).
Results: 135 patients were randomized (54 patients in the intradermal lignocaine group, 54 patients in the subcutaneous lignocaine group, and 27 in the subcutaneous saline group). The mean patient-assessed pain score for the intradermal lignocaine group was 1.8 (+/- 1.1), which was a relative reduction of 47% (95% C.I. 31%-59%, p < 0.0001) from the mean patient-assessed pain score of 3.4 (+/- 1.1) for the subcutaneous saline group. The mean patient-assessed pain score for the subcutaneous lignocaine group was 2.1 (+/- 1.1), which was also a significant relative reduction of 36% (95% C.I. 17%-51%, p = 0.0001) compared to the subcutaneous saline group. Intradermal lignocaine reduced pain more than subcutaneous lignocaine, with a relative pain reduction difference of 20% (95% CI -4%-49%, p = 0.05). Bruising was more frequent in the subcutaneous lignocaine group (9.3%) than in the intradermal (0%) and saline groups (0%).
Conclusion: Intradermal lignocaine is at least as effective as subcutaneous lignocaine for reducing patient-perceived pain from arterial blood gas sampling and results in less bruising.
Arterial blood gas (ABG) sampling is a common investigative procedure that provides essential information on oxygenation, ventilation, and acid-base balance. This information has wide-ranging applications such as the care of critically ill patients on ventilatory support as well as assessing outpatients with respiratory comorbidities for the provision of domiciliary oxygen therapy [1,2]. ABG sampling requires arterial puncture in addition to venepuncture for routine investigations. The associated pain can be severe and unpleasant, presenting a barrier for patients and clinicians to perform this procedure [2,3].
Several studies have demonstrated that local anesthesia (LA) prior to ABG sampling reduces the pain associated with the procedure and the use of LA is recommended by multiple international groups, including the American Association of Critical Care Nurses and the British Thoracic Society [4-7].
Despite the recommendations for using LA before ABG sampling, surveys have shown limited uptake of this in actual practice settings [1,6,8]. Several reasons have been given for not using LA including concerns that LA can obscure the anatomy of the ABG puncture site making sampling more difficult, potential inadvertent arterial injection of LA, and resource or access problems obtaining LA [1,6]. However, by far the most common reason is the prevailing perception that ABG sampling does not require LA for pain reduction [2,8].
Many different routes of LA administration before ABG sampling are available, but the optimal method is not well defined as recommendations for the route, volume, and type of LA vary across the medical literature. Most studies demonstrating the efficacy of LA for ABG sampling pain reduction have used subcutaneous mepivacaine or lignocaine infiltration, which is reflected in most guidelines [3,4,9,10]. Multiple studies have demonstrated the ineffectiveness of topical anesthetic creams for this purpose, yet there remain other effective LA types and administration routes including ethyl chloride spray, 10% lignocaine spray, and cryo-analgesia techniques [9-14]. However, no study to date has evaluated the differences between intradermal and subcutaneous routes of lignocaine infiltration, which is the primary objective of this trial.
Study design
We undertook a randomized controlled trial at Middlemore Hospital in Auckland, New Zealand in 2011. Patients attending a nurse-led outpatient oxygen clinic who were 18 to 90 years of age, had an oxygen saturation of 93% or less at rest, and required an ABG for domiciliary oxygen assessment were eligible for inclusion. All included patients had provided written informed consent. Exclusion criteria were allergy to lignocaine, anatomical distortion of the wrist, infection at the site of ABG sampling, and inability to complete a questionnaire. The trial was approved by the National Ethics Advisory Committee (NTY/10/03/028). The primary aim of this study was to evaluate the differences in perceived pain between intradermal lignocaine, subcutaneous lignocaine, and a subcutaneous saline placebo-control group. The secondary aims were to evaluate if different routes of LA administration had an impact on the difficulty and complications of ABG sampling.
Demographic variables collected were age, gender, ethnicity, weight, and wrist circumference. Other baseline variables that may be potential confounders were recorded and include analgesia use, previous ABG sampling experience, and patients’ recollected pain scores from their experience. The primary outcome measure was patient-assessed pain scores using a graphic rating scale (GRS) of zero (no pain) to ten (worst pain ever) (Figure 1) [15]. The GRS was used in our study due to the multilinguistic nature of our study population. Secondary outcome measures included nurse-assessed pain scores, level of difficulty obtaining ABG rated by the primary investigator (both using the GRS), and adverse events resulting from the combined LA infiltration and ABG sampling.
Figure 1: Graphic rating scale - zero (no pain) to ten (worst pain ever).
Randomization and blinding
Patients were randomized via computer-generated program into 3 study groups to receive intradermal 1% lignocaine, subcutaneous 1% lignocaine, or subcutaneous normal saline prior to ABG sampling. The randomization of the respective three groups occurred in a 2:2:1 ratio with a permuted block size of 4. This was designed with the intention of giving more statistical power to detect differences between the intradermal and subcutaneous lignocaine groups. Patients and nurse assessors were blinded to the assigned infiltration by having an opaque box covering the patient’s ABG sampling site. However, the primary investigator who performed all the infiltrations and ABG sampling as well as the statistician were not blinded.
ABG sampling protocol
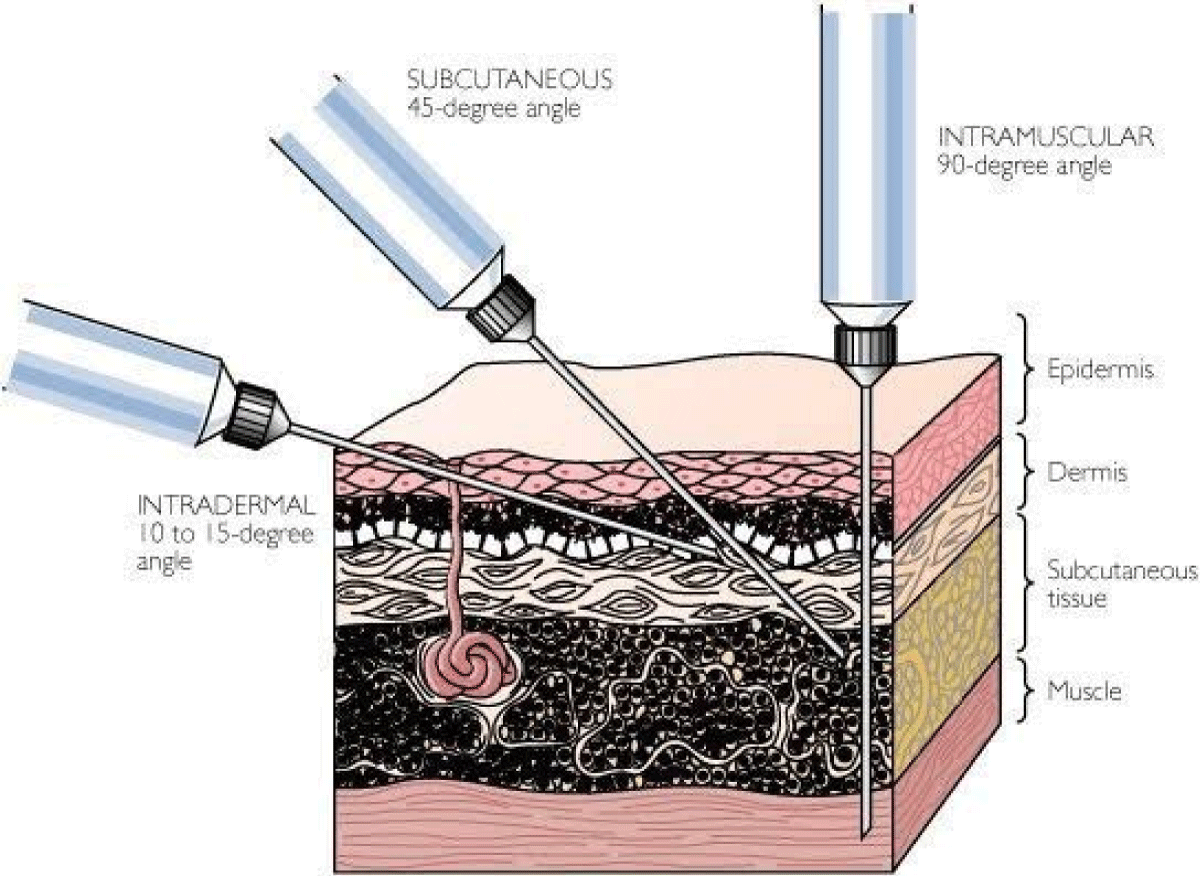
LA infiltrations were administered with 0.25 ml 1% lignocaine or normal saline using 25-gauge needles. For intradermal infiltrations, the needles with the bevel up were inserted into the dermis at an angle of 5-15 degrees. For subcutaneous infiltrations, the needles with the bevel up were directed into the subcutaneous tissue at an angle of about 45 degrees (Figure 2) [16]. ABG sampling was performed 2 minutes after LA infiltration, using a 23-gauge needle inserted into the radial artery in accordance with best standard practice [17]. After the procedure, the pain experienced was assessed by the patients in the absence of the primary investigator who performed the procedure. The nurse assessor also visually assessed the pain experienced by the patients. All LA infiltration and ABG sampling were performed by the primary investigator to minimize any variation in technique that could influence pain perception. The same nurse assessor was used to assess all the pain scores.
Figure 2: Lignocaine anesthesia showing intradermal and subcutaneous techniques.
Statistical analysis
Unadjusted geometric means of the patient-assessed pain score were reported with standard deviation (SD), median, and interquartile range (IQR). Analysis of covariance was used to assess for any significant differences between patient-assessed pain scores across the three groups, adjusted for age, waist circumference, gender, ethnicity, analgesia use, and patient pain scores from past experiences. The patient-assessed pain scale was log-transformed in the analysis.
Nurse-assessed pain scores and level of difficulty obtaining ABG were analyzed as ordinal responses. A simple proportional odds model (POM) for ordinal responses was used to derive the odds ratios. Multiple POM models were applied to adjust for age, waist circumference, gender, ethnicity, analgesia use, and patient pain scores from past experiences in the final analysis. Nurse-assessed pain scores and level of difficulty obtaining ABG were grouped when the scores were three or greater. This grouping process ensured the proportional odds assumption was valid in the final POM model. Chi-square tests were used to assess if there was any difference in the distribution of adverse events across the three infiltration groups. All analyses were based on intention to treat approach.
SAS 9.3 software (released by SAS Institute Inc., Cary, NC, USA.) and R version 2.15.0 (Released by (https://www.r-project.org/) were used in the randomization and analysis.
Adverse events monitoring
The data safety monitoring board reviewed adverse events and efficacy. A safety stopping rule was set at the interim analysis, such that if a severe adverse event in any of the active intervention groups was significantly higher than the normal practice (p < 0.05), the active treatment was considered for termination.
Demographic and baseline variables
A total of 135 patients were included in the trial. Demographic characteristics of the patients, including age, gender, ethnicity, waist circumference, and weight were similar across the three study groups without any statistically significant differences. Patients’ experiences of previous ABG sampling and the associated pain scores were also similar across the study groups. Two patients in the intradermal lignocaine group and 2 in the placebo group took analgesic medication (aspirin) before ABG sampling (Table 1).
| Table 1: Patient Demographic and Baseline Variables. | |||
| Intradermal Lignocaine | Subcutaneous Lignocaine | Subcutaneous Saline | |
| n = 54 | n = 54 | n = 27 | |
| Age | 66 (14) | 67 (13) | 68 (13) |
| Wrist circumference (cm) | 18.3 (2.0) | 18.5 (2.3) | 18.6 (2.0) |
| Weight (kg) | 91.1 (40.2) | 94.5 (38.7) | 98.0 (39.5) |
| Gender (Female) | 26 (48.2%) | 31 (57.4%) | 15 (55.6%) |
| Ethnicities | |||
| Maori | 20 (37.0%) | 8 (14.8%) | 5 (18.5%) |
| Pacific Islander | 9 (16.7%) | 18 (33.3%) | 7 (25.9%) |
| Caucasian | 21(38.9%) | 23 (42.6%) | 14 (51.9%) |
| Others | 4 (7.4%) | 5 (9.3%) | 1 (3.7%) |
| Analgesia Use | 2 (4%) | 0 (0%) | 2 (7%) |
| Previous ABG experience | 47 (87.0%) | 52 (96.3%) | 23 (85.2%) |
| Pain score for previous ABG | 5.5 (3.1) | 6.2 (3.2) | 5.8 (2.7) |
| Pain score for previous ABG (median (IQR)) | 5.5 (3.0, 8.0) | 7.0(4.0, 9.0) | 5.0 (4.0, 8.0) |
| *Continuous variables are presented as mean (STD) or median (IQR) and categorical variables are presented as count (%). | |||
Patient-assessed pain scores
Both intradermal and subcutaneous lignocaine infiltration reduced patient-assessed pain scores significantly compared to subcutaneous saline (Tables 2,3). The mean patient-assessed pain score for the intradermal lignocaine group was 1.8 (+/- 1.1), which was a relative reduction of 47% (95% C.I. 31%-59%, p < 0.0001) from the mean patient-assessed pain score of 3.4 (+/- 1.1) for the subcutaneous saline group after adjusting for confounding variables (age, wrist circumference, gender, ethnicity, analgesia use, and patient pain scores from past experiences). The mean patient-assessed pain score for the subcutaneous lignocaine group was 2.1 (+/- 1.1), which was also a significant relative reduction of 36% (95% C.I. 17% - 51%, p = 0.0001) compared to the subcutaneous saline group after adjusting for confounders.
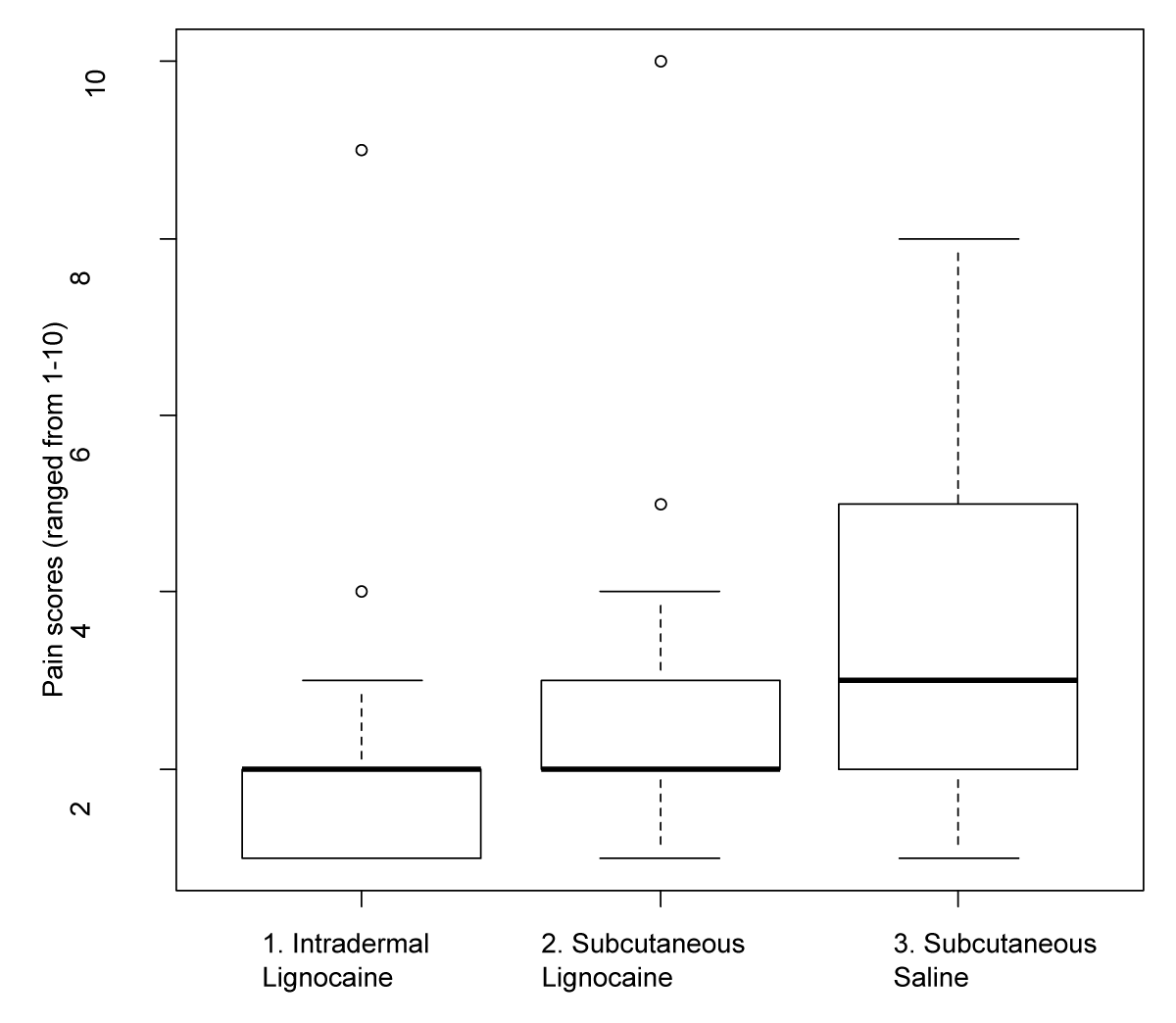
When comparing the two administration routes for LA, intradermal lignocaine reduced pain more than subcutaneous lignocaine, with a relative pain reduction difference of 20% (95% CI -4%-49%, p = 0.05) (Table 3). The comparative distribution of patient-assessed pain scores between the three infiltration groups is shown as a box and whisker plot in Figure 3.
Figure 3: Comparative patient-assessed pain scores relative to infiltration groups.
Nurse-assessed pain scores
Similar findings were observed for nurse-assessed pain scores where intradermal and subcutaneous lignocaine groups had significantly lower scores compared to the subcutaneous saline group. The proportions of patients with nurse-assessed pain scores greater than or equal to 3 were 4%, 11%, and 44% for intradermal lignocaine, subcutaneous lignocaine, and subcutaneous saline respectively (Table 2). The nurse-assessed pain scores had a significant positive correlation with the patient-assessed pain scores (Spearman correlation coefficient of 0.70, p < 0.0001). Patients with intradermal lignocaine before the ABG sampling were less likely to have higher pain scores than patients with subcutaneous saline (odds ratio 0.08, 95% C.I. 0.03-0.21, p < 0.0001); patients with subcutaneous lignocaine were also less likely to receive higher pain scores than patients with subcutaneous saline (odds ratio 0.15, 95% C.I. 0.06-0.37, p < 0.0001) (Table 3).
| Table 2: Summary of Primary and Secondary Outcome Variables. | ||||
| Intradermal Lignocaine | Subcutaneous Lignocaine | Subcutaneous Saline | p -value | |
| Patient assessed pain score** (range 0-10) (mean (SD)) | 1.8 (1.1) | 2.1 (1.1) | 3.4 (1.1) | < 0.0001* |
| Patient assessed pain score (range 0-10) (median (IQR)) | 2.0 (1.0, 2.0) | 2.0 (2.0, 2.0) | 3.0 (2.0, 5.0) | |
| The nurse assessed the pain score | ||||
| 1 | 39(72%) | 32(59%) | 5(19%) | |
| 2 | 13(24%) | 16(30%) | 10(37%) | |
| ≥ 3 | 2(4%) | 6(11%) | 12(44%) | < 0.0001‡ |
| Level of difficulty obtaining ABG | ||||
| 1 | 40(74%) | 36(67%) | 20(74%) | |
| 2 | 10(19%) | 9(17%) | 6(22%) | |
| ≥ 3 | 4(7%) | 9(17%) | 1(4%) | 0.45‡ |
| Adverse events | ||||
| Bruising | 0(0%) | 5(9.3%) | 0(0%) | |
| Tenderness | 1(2%) | 1(2%) | 0(0%) | 0.05† |
| *F test in one way Analysis of variance; **Unadjusted Geometric means of the current pain score; ‡Wald chi square test using proportional odds model for ordinal responses; †Fisher’s Exact Test | ||||
| Table 3: Pair-wise comparison in outcomes between different study groups. | |||
| Intradermal Lignocaine vs. Subcutaneous Saline | Subcutaneous Lignocaine vs. Subcutaneous Saline | Subcutaneous Lignocaine vs. Intradermal Lignocaine | |
| Relative patient assessed pain reduction (95% C.I) and p - value** |
47% (95% C.I. 31%, 59%) p < 0.0001 | 36% (95% CI, 17%, 51%), p = 0.0001 | -20% (-49%, 4%) P = 0.05 |
| The odds ratio of having higher nurse-assessed pain scores (95% C.I.) and p - value‡ |
0.08 (95% C.I. 0.03, 0.21) P < 0.0001 |
0.15 (95% C.I. 0.06, 0.37), p < 0.0001 | 1.88 (95% C.I. 0.85, 4.2) p = 0.12 |
| The odds ratio of a higher level of difficulty obtaining ABG (95% C.I.) and p - values ‡ |
1.05(0.36, 3.02) P = 0.93 |
1.63(0.58, 4.55) P = 0.35 |
1.55(0.69, 3.52) P = 0.29 |
| Difference in proportion of adverse events (95% C.I.) and p - value* |
1.9% (-1.7%, 5.4%) P > 0.9 |
11.1% (2.7%, 19.5%) p = 0.35 | 9.3% (0.1%, 18.4%) P = 0.11 |
| ** F test in one-way Analysis of variance, ‡ Wald chi-square test using proportional odds model for ordinal responses, * Fisher’s Exact Test | |||
Level of Difficulty Obtaining ABG
Using lignocaine in either administration route did not significantly affect the difficulty of obtaining ABG compared to subcutaneous saline. However, it is worth noting that the subcutaneous lignocaine group had a higher portion of patients rated with the level of difficulty at three or above (17%) compared to the other two groups although this did not reach statistical significance overall (Table 2).
Adverse events
Adverse events reported in this trial were either bruising or tenderness. The subcutaneous lignocaine group reported more adverse events than the intradermal lignocaine and subcutaneous saline groups (p = 0.05). This was due solely to all bruising reports arising from the subcutaneous lignocaine group (9.3%) and none from the other two groups.
The results of this study demonstrate that LA with lignocaine injected by both intradermal and subcutaneous routes prior to ABG sampling reduced both patient and nurse-assessed pain scores compared to placebo. This is consistent with most trials related to this subject and reflects best practice recommendations in clinical guidelines [3,4,9,10]. In addition, our trial found that intradermal lignocaine reduced pain associated with ABG sampling more than subcutaneous lignocaine, the significant statistical threshold was almost reached (p = 0.05).
The medical literature on local anesthesia favors subcutaneous infiltration to be less painful than the intradermal route and this is likely founded on the basis that most nociceptive nerve endings are in the dermal layer [18]. However, there are no trials to substantiate this theory other than anecdotal evidence [19,20]. Our study results contradict this notion and argue that direct infiltration to the dermal layer leads to an improved local anesthetic effect. This may have been due to rapid and more complete anesthesia of nociceptive nerve endings in the dermal layer and less injury to tissue with a deeper subcutaneous injection. Other factors that have been reported to reduce pain perception in LA infiltration include the bevel direction, gauge of the needle, pH, and concentration of lignocaine but these factors were controlled in all our study groups [16,20-22]. In addition, we used a small volume (0.25 ml) of lignocaine and this may have reduced the amount of mechanical or stretch-related nociception.
Previous studies have demonstrated LA infiltration does not increase ABG sampling difficulty [8]. Our study supports this finding, and most samples were taken with a low level of difficulty. The only adverse event significantly different between the study groups was bruising, which occurred exclusively in the subcutaneous group and is likely explained by the vascularity of the subcutaneous layer as well as the vasodilatory effects of lignocaine [23]. This again supports the intradermal route as being a superior method of lignocaine infiltration.
In this study, the primary investigator carried out all the LA, and placebo infiltrations as well as the ABG sampling for the 135 participants. This eliminated variable operator experience between the groups and is a strength of this study. The primary investigator was an experienced oxygen clinic nurse specialist and the significant pain reduction provided by LA use in this study should dispel the myth that ABG sampling performed by experienced personnel does not require LA [24]. It is worthwhile pointing out that given this study was conducted in an outpatient oxygen clinic setting, the results are less applicable to emergency settings where variable operator experience and the pressure of time are significant factors to consider. Nevertheless, multiple other studies conducted in emergency settings have validated the effectiveness of LA before ABG sampling [10,13,14].
Other potential confounders that may influence pain perception in this study include gender and pain scores for previous ABG experience but neither of these factors were significantly different between the study groups [25]. Our study population had a substantial proportion of Māori and Pacific patients given the New Zealand context and more Māori participants were in the intradermal group relative to the other groups. Although we cannot exclude a difference in pain perception between Māori and other ethnic groups, this is not supported by the finding of reduced pain in the subcutaneous group compared to the placebo group, which had a higher proportion of Māori than the subcutaneous group [26]. Weight and wrist circumference were also likely higher in our study population due to the prevalence of obesity in the Counties Manukau district [27]. However, our statistical analysis adjusted for these factors as well as for ethnicity.
Various other methods of LA have proven to be effective before ABG sampling in other studies, notably ethyl chloride and lignocaine sprays [13,14]. Both are non-invasive compared to lignocaine infiltration but are also more costly. On the other hand, topical anesthetic creams have been well-established to be ineffective in reducing pain associated with ABG sampling [9-12]. In the study by Pagnucci et al. cryoanalgesia using a bag of ice applied over the ABG sampling site for 3 minutes has so far been the most cost-effective way of providing LA albeit overall slightly less effective than mepivacaine infiltration [10]. Future studies comparing all these LA methods with intradermal lignocaine infiltration and their individual cost-effectiveness will be informative.
LA administration before ABG sampling remains the best practice for pain reduction and should be the standard of care. The intradermal route of lignocaine infiltration is effective and associated with fewer adverse events than the subcutaneous route.
- Sado DM, Deakin CD. Local anaesthesia for venous cannulation and arterial blood gas sampling: are doctors using it? J R Soc Med. 2005 Apr;98(4):158-60. doi: 10.1177/014107680509800405. PMID: 15805556; PMCID: PMC1079439.
- Hudson TL, Dukes SF, Reilly K. Use of local anesthesia for arterial punctures. Am J Crit Care. 2006 Nov;15(6):595-9. PMID: 17053266.
- Giner J, Casan P, Belda J, González M, Miralda RM, Sanchis J. Pain during arterial puncture. Chest. 1996 Dec;110(6):1443-5. doi: 10.1378/chest.110.6.1443. PMID: 8989058.
- Guidelines for the measurement of respiratory function. Recommendations of the British Thoracic Society and the Association of Respiratory Technicians and Physiologists. Respir Med. 1994 Mar;88(3):165-94. PMID: 8209067.
- Lynn-McHale Wiegand DJ, Carlson KK. AACN Procedure Manual for Critical Care. Elsevier/Saunders. 2005; https://books.google.co.nz/books/about/AACN_Procedure_Manual_for_Critical_Care.html?id=CyJtAAAAMAAJ&redir_esc=y
- Proehl JA. Emergency Nursing Procedures. Elsevier/Saunders. 2004; https://books.google.co.nz/books/about/Emergency_Nursing_Procedures.html?id=xTVtAAAAMAAJ&redir_esc=y
- Bates D, Cutting P. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Local anaesthetic and arterial puncture. Emerg Med J. 2001 Sep;18(5):378. doi: 10.1136/emj.18.5.378. PMID: 11559614; PMCID: PMC1725663.
- Lightowler JV, Elliott MW. Local anaesthetic infiltration prior to arterial puncture for blood gas analysis: a survey of current practice and a randomised double blind placebo controlled trial. J R Coll Physicians Lond. 1997 Nov-Dec;31(6):645-6. PMID: 9409498; PMCID: PMC5421072.
- Giner J, Casan P, Belda J, Litvan H, Sanchis J. Utilización de la crema anestésica EMLA en la punción arterial [Use of the anesthetic cream EMLA in arterial punction]. Rev Esp Anestesiol Reanim. 2000 Feb;47(2):63-6. Spanish. PMID: 10769553.
- Pagnucci N, Pagliaro S, Maccheroni C, Sichi M, Scateni M, Tolotti A. Reducing Pain During Emergency Arterial Sampling Using Three Anesthetic Methods: A Randomized Controlled Clinical Trial. J Emerg Med. 2020 Jun;58(6):857-863. doi: 10.1016/j.jemermed.2020.03.027. Epub 2020 Apr 27. PMID: 32354590.
- Tran NQ, Pretto JJ, Worsnop CJ. A randomized controlled trial of the effectiveness of topical amethocaine in reducing pain during arterial puncture. Chest. 2002 Oct;122(4):1357-60. doi: 10.1378/chest.122.4.1357. PMID: 12377864.
- Aaron SD, Vandemheen KL, Naftel SA, Lewis MJ, Rodger MA. Topical tetracaine prior to arterial puncture: a randomized, placebo-controlled clinical trial. Respir Med. 2003 Nov;97(11):1195-9. doi: 10.1016/s0954-6111(03)00226-9. PMID: 14635973.
- Cakmak F, Gur A. Ethyl chloride spray, a local anesthetic in arterial blood gas sampling: A randomized, controlled, double-blinded study. Am J Emerg Med. 2022 Sep;59:63-66. doi: 10.1016/j.ajem.2022.06.038. Epub 2022 Jun 22. PMID: 35797844.
- Yıldız İU, Yıldırım Ç, Özhasenekler A, Şener A, Gökhan Ş. Effectiveness of lidocaine spray on radial arterial puncture pain: A randomized double-blind placebo controlled trial. Am J Emerg Med. 2021 Dec;50:724-728. doi: 10.1016/j.ajem.2021.09.077. Epub 2021 Oct 1. PMID: 34879493.
- Ekblom A, Hansson P. Pain intensity measurements in patients with acute pain receiving afferent stimulation. J Neurol Neurosurg Psychiatry. 1988 Apr;51(4):481-6. doi: 10.1136/jnnp.51.4.481. PMID: 3259976; PMCID: PMC1032956.
- Candiotti K, Rodriguez Y, Koyyalamudi P, Curia L, Arheart KL, Birnbach DJ. The effect of needle bevel position on pain for subcutaneous lidocaine injection. J Perianesth Nurs. 2009 Aug;24(4):241-3. doi: 10.1016/j.jopan.2009.04.003. PMID: 19647661.
- Dev SP, Hillmer MD, Ferri M. Videos in clinical medicine. Arterial puncture for blood gas analysis. N Engl J Med. 2011 Feb 3;364(5):e7. doi: 10.1056/NEJMvcm0803851. PMID: 21288091.
- Hsu DC, Stack AM, Walls RM, Miller SJ, Ganetsky M. Subcutaneous infiltration of local anesthetics. Uptodate. Cited 18 June 2023. https://www.uptodate.com/contents/subcutaneous-infiltration-of-local-anesthetics.
- Scarborough D, Bisaccia E, Schuen W, Swensen R. Anesthesia for the dermatologic surgeon. Int J Dermatol. 1989 Dec;28(10):629-37. doi: 10.1111/j.1365-4362.1989.tb02431.x. PMID: 2592127.
- Quaba O, Huntley JS, Bahia H, McKeown DW. A users guide for reducing the pain of local anaesthetic administration. Emerg Med J. 2005 Mar;22(3):188-9. doi: 10.1136/emj.2003.012070. PMID: 15735267; PMCID: PMC1726707.
- Palmon SC, Lloyd AT, Kirsch JR. The effect of needle gauge and lidocaine pH on pain during intradermal injection. Anesth Analg. 1998 Feb;86(2):379-81. doi: 10.1097/00000539-199802000-00030. PMID: 9459252.
- Long CC, Motley RJ, Holt PJ. Taking the 'sting' out of local anaesthetics. Br J Dermatol. 1991 Nov;125(5):452-5. doi: 10.1111/j.1365-2133.1991.tb14771.x. PMID: 1751351.
- Guinard JP, Carpenter RL, Morell RC. Effect of local anesthetic concentration on capillary blood flow in human skin. Reg Anesth. 1992 Nov-Dec;17(6):317-21. PMID: 1286052.
- Theodore AC, Manaker S, Finlay G. Arterial blood gases. Uptodate. Cited 20 August 2023. 2023. https://www.uptodate.com/contents/arterial-blood-gases
- Rosseland LA, Stubhaug A. Gender is a confounding factor in pain trials: women report more pain than men after arthroscopic surgery. Pain. 2004 Dec;112(3):248-253. doi: 10.1016/j.pain.2004.08.028. PMID: 15561379.
- Mcgavock ZC, Barnes HM, Mccreanor T. Māori and pain: A literature review. AlterNative 8(2): 163–175. https://www.researchgate.net/publication/313782693_Maori_and_pain_A_literature_review
- Bradley K, Cowan S, Babor R, Morrow J, MacCormack A, Rahiri JL, Murphy R. Investigating the distribution of primary and secondary care referrals for public-funded bariatric surgery at Counties Manukau Health (CMH). N Z Med J. 2023 Jun 16;136(1577):65-75. PMID: 37778320.