More Information
Submitted: January 30, 2024 | Approved: February 27, 2024 | Published: February 28, 2024
How to cite this article: Claudio C, Felix SM, Luciana PC, Veronica A, Belen LM, et al. Effect of Azithromycin on Lung Function and Pulmonary Exacerbations in Children with Post-infectious Bronchiolitis Obliterans. A Double-blind, Placebo-controlled Trial. J Pulmonol Respir Res. 2024; 8: 009-014.
DOI: 10.29328/journal.jprr.1001052
Copyright License: © 2024 Claudio C, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Effect of Azithromycin on Lung Function and Pulmonary Exacerbations in Children with Post-infectious Bronchiolitis Obliterans. A Double-blind, Placebo-controlled Trial
Castaños Claudio1*, Salin Maximiliano Felix2, Pereyra Carla Luciana3, Aguerre Veronica2, Lucero Maria Belen2, Bauer Gabriela4, Zylbersztajn Brenda5, Leviled Leonor6 and Gonzalez Pena Hebe2
1Pulmonology Department, Hospital Nacional de Pediatría “Juan P. Garrahan”, Combate de los Pozos 1881, Argentina
2Pulmonology Department, Hospital Nacional de Pediatría “Juan P. Garrahan”.Buenos Aires, Argentina
3Pediatrics, Hospital Italiano de Bueno Aires, Buenos Aires, Argentina
4Hospital Nacional de Pediatría “Juan P. Garrahan”, Buenos Aires, Argentina
5PharmD Pediatrics, Clinica las Condes, Santiago de Chile, Chile
6Nurse, Hospital Nacional de Pediatría “Juan P. Garrahan”, Buenos Aires, Argentina
*Address for Correspondence: Castaños Claudio, Pulmonology Department, Hospital Nacional de Pediatría “Juan P. Garrahan”, Combate de los Pozos 1881, Argentina, Email: [email protected]
Introduction: Acute lower respiratory infection (ALRI) of viral etiology is a frequent consultation in pediatrics. Post-infectious bronchiolitis obliterans (PIBO) is a rare and potentially severe disorder following ALRI, characterized by partial or complete obstruction of the small airways by inflammatory tissue. There is evidence that macrolides reduce morbidity and mortality in diffuse panbronchiolitis, which may have similar inflammatory and obstructive components.
We hypothesized that the effect of azithromycin (AZ) may improve lung function and reduce pulmonary exacerbations in PIBO.
Methods
Study design: A double-blind, randomized, placebo-controlled trial.
Patients: We enrolled patients with PIBO followed-up at the Pulmonology department between 5 years to 18 years.
Treatment regimen: The patients were randomized to receive active drug or placebo three times a week.
Clinical evaluation: Clinical evaluation, pulse oximetry, lung function, and 6-min walk test were performed before and after study initiation and at 1, 3, and 6 months.
CT scan and a quality of life questionnaire were performed at the beginning and the end of the Study.
Results: 29 patients, 15 in G1 (10 males) and 14 in G2 (7 males) were included.
There were no significant differences in FVC, FEV1, TLC, RV, or sGaw between the treatment group and controls. In addition, no significant differences were observed in exacerbations, quality of life questionnaire, or HRCT scan scores.
Conclusion: No differences were observed between the groups. Further studies are necessary to allow us to find a better treatment, as azithromycin does not seem to be efficacious.
Acute lower respiratory infection (ALRI) of viral etiology is one of the main reasons for consultation in pediatric healthcare. Among the different types of ALRI, bronchiolitis and pneumonia are the most common [1].
Post-infectious bronchiolitis obliterans (PIBO) is a rare and potentially severe disorder following ALRI. PIBO is characterized by partial or complete obstruction of the small airways by inflammatory tissue.
Subsequent repair includes scar proliferation and metaplasia of the bronchial epithelium, necrotic tissue organization that occupies the airway lumen with granulation tissue, vascular remodeling, and infiltration of lipid-laden macrophages and chronic inflammatory cells.
The severity of ALRI is associated with various risk factors in children, their families, and their social environment, particularly with the aggressive nature of certain viral agents [1,2]. Adenovirus (Ad) is by far the most common cause of PIBO and is associated with a high mortality rate at least in Argentinian population [3 ].
There is evidence that macrolides reduce the morbidity and mortality in diffuse panbronchiolitis in the Japanese population. Diffuse panbronchiolitis has an inflammatory and obstructive component similar to bronchiolitis obliterans [4,5]. Macrolides have also been used in patients with cystic fibrosis (CF) undergoing a severe endobronchial inflammatory process and in patients with bronchiolitis obliterans after lung transplantation resulting in decreased morbidity, significant improvement in lung function, and longer survival [6-8].
We hypothesized that the potential immunomodulatory and anti-inflammatory effect of the macrolide azithromycin (AZ) may improve lung function and reduce pulmonary exacerbations in pediatric patients with PIBO.
Study design
A double-blind, randomized, placebo-controlled trial (NCT05299567) was conducted, between June 2010 and December 2011 .
Patients were evaluated at baseline and at 1 (28 days), 3 (84 days), and 6 (168 days) months of treatment.
Patients
We enrolled patients with PIBO followed-up at the Department of Pediatric Pulmonology of the Pediatric Hospital “Juan P. Garrahan”, between 5 years and 18 years of age, who were able to do the pulmonary function test and had been clinically stable (without cough, secretions, or wheezing on auscultation and stable pulmonary function) for three weeks prior to the study.
PIBO was defined as the presence of signs and symptoms of chronic lung disease (digital clubbing, rigid chest, persistent coughing, and wheezing) associated with bronchial obstruction without response to bronchodilators and CT scan images showing air trapping and hypoperfusion in a mosaic pattern.
Patients were excluded from the study if they were not able to perform the pulmonary function test or had any other chronic lung disease (bronchopulmonary dysplasia or CF) primary or secondary immunodeficiency, heart disease, history of portal hypertension, liver cirrhosis, splenomegaly, abnormal laboratory tests at baseline, or history of hypersensitivity to macrolides.
The patients were randomized to two treatment arms. One group (the intervention group) received azithromycin, while the other group (the control group) received a placebo on alternate days for 6 months.
Treatment regimen
Azithromycin was administered in capsules of 250 mg. Both the drug and placebo were prepared in equal size, shape, and color at the hospital pharmacy. Patients weighing less than 40 kg received one capsule and those weighing more than 40 kg two capsules three times a week of active drug or placebo according to randomization.
Patients were randomized by the pharmacy staff in blocks of four patients through a telephone center contacted by the investigator once the patient was enrolled. In each block, two patients received the active drug and two placebo.
Clinical evaluation
Weight, height, blood pressure, pulse oximetry, spirometry, lung volume measurement, and 6-min walk test were performed before and after study initiation and at 1, 3, and 6 months of treatment. A CT scan was performed and a quality of life questionnaire (PedsQL) was administered at the beginning and the end of the study. In those under 12 years of age it was on Parent Proxy Report and in those older was Child Self-Report .
Primary outcome measures were forced vital capacity (FVC) and forced expiratory volume in the first second (FEV1). The following lung volumes were obtained: total lung capacity (TLC), residual volume (RV), TLC/RV ratio, and specific airway conductance. All testing was performed on a Collins Raptor CPL pulmonary function test equipment. Forced vital capacity (FVC, FEV1, and FEF 25% - 75%) and flow-volume curves were recorded according to the American Thoracic Society guidelines by a trained technician [9].
Normal FVC and FEV1 values were defined as values greater than 80% of the reference values for sex, age, and height. Reference equations by Polgar [10] were used for children up to 12 years and by Knudson [11] for children older than 12 years.
The 6-minute walk test was done on a flat surface, controlling pulse oximetry and heart rate at study initiation and every minute thereafter.
At each visit, the number of respiratory illnesses, use of bronchodilators, oral or intravenous ATB, emergency department (ED) visits, or need for hospital admission were evaluated.
Laboratory blood studies as well as liver and kidney function tests were performed at study entry, one month after treatment initiation, and at study completion to monitor adverse effects.
Patients who did not complete the protocol were withdrawn from the study and data obtained thus far were analyzed on an intention-to-treat basis.
To assess the quality of life, PedsQL Version 4.0 was used.
A chest high-resolution CT scan (HRCT) was performed on a GE LightSpeed 64-slice CT Scanner.
Axial cuts of 1.2 mm thickness were obtained during inspiration at intervals of 10 mm in young children and 20 mm in older children and four cuts during expiration at the levels of the aortic arch and the tracheal carina. Half the cardiac silhouette and the diaphragmatic cupula were also evaluated. The images were viewed at a window level of –700 Hounsfield units (HU) and a window width of 1,500 HU.
CT scans were performed at study initiation and at the end of the study to evaluate structural changes in the lungs. And analyzed by a trained pediatric radiologist who was blinded to the clinical and functional data. A modified Brody and Bhalla composite HRCT scan score was used for patients with primary ciliary dyskinesia [12]. The score is based on the description of five features (bronchiectasis, mucous plugging, peri-bronchial thickening, parenchymal abnormalities, and lung hyperinflation) in six lung regions. Each lung is divided into three regions:
(1) Upper region: From the apex to the carina
(2) Middle region: From the carina to the inferior pulmonary veins
(3) Lower region: From the inferior pulmonary vein to the base
Each region was assigned a score: 0 points if no and 1 point each if the following findings were present: mucous plugging, peri-bronchial thickening, parenchymal abnormalities (condensation and collapse), and lung hyperinflation. The different degrees of severity were evaluated compared to the diameter of the adjacent pulmonary artery (APA) as proposed in the score by Bhalla and Brody: 1 point for mild bronchiectasis (1-2 times the diameter of the APA), 2 points for moderate bronchiectasis (2-3 times the diameter of the APA), and 3 points for severe bronchiectasis points (up to 3 times the diameter of the APA).
A history of lung surgery was also considered: 5 points for lobectomy and 2 points for partial lobectomy. The score ranged from 0 to 42 points with no points awarded for surgery.
Adverse effects were divided into mild and severe.
The study was approved by the Hospital Ethics Committee and all patients or parents/caregivers signed the written informed assent and consent form prior to the study.
Outcomes measures
Primary outcome: To assess the effect of azithromycin compared to placebo on lung function measured by FEV1 and FVC over a 52-week treatment period.
Secondary outcomes: To assess the effect of azithromycin compared to placebo in the number of exacerbations and requirement of oral or intravenous (IV) antibiotics (ATB),
Quality of life, and changes in the CT scan pre- and post-treatment.
Evaluation of the tolerability and safety of the medication.
Statistical analysis
Data analysis was performed on an intention-to-treat basis.
It was estimated that at least 22 patients were required for each study arm to achieve an effect size of 0.10 for a change of 5% in lung function, which was considered relevant, with an alpha of 0.05 and a beta error of 0.20.
Comparisons between the two arms were analyzed using the t-test for independent data or the Wilcoxon rank sum test as appropriate in all cases. Data are presented as summaries of position and dispersion measures. The results are presented as relative risk and 95% confidence interval (CI). A p < 0.05 was considered significant.
Statistical analysis was performed with SPSS version 10.0.
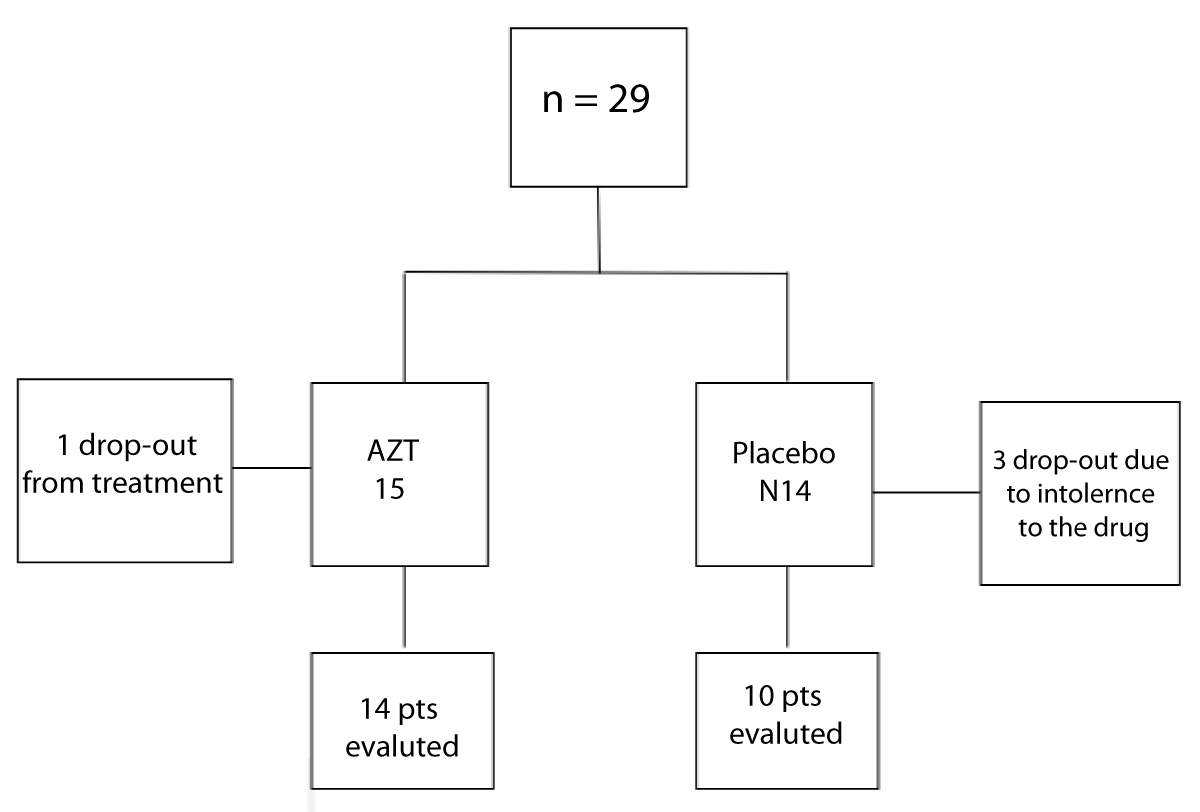
Twenty-nine patients were included, 15 in G1 (intervention group) (10 males) with a mean age of 13.4 ( ± 2.6) years and 14 in G2 (placebo group) (7 males) with a mean age of 14 ( ± 2.5) years (p = 0.38). Five patients did not complete the protocol. Four patients abandoned the study; one from G1 and three from G2. One patient from G2 showed intolerance to the drug and was withdrawn from the study (Figure 1).
Figure 1: Study sample diagram.
Twenty-four patients were included for analysis: 14 in G1 and 10 in G2. There were no differences in pulmonary function test (PFT) and age, sex, weight, and height between the groups (Figure 1).
Baseline PFT in G1 was the following: FVC% 79.14 ± 18.89; FEV1% 52.42 ± 17.39; TLC% 112.64 ± 12.67; RV% 215.5 ± 51.42, while baseline PFT in G2 was: FVC% 82.7 ± 22.57; FEV1% 58.5 ± 23.80; TLC% 1114.12 ± 19.46; RV% 198.5 ± 70.46; (p = NS) (Table 1).
| Table 1: Baseline characteristics of the randomized patients. | |||
| Group 1 Azithromycin (n = 14) |
Group 2 Placebo (n = 10) |
p - value** | |
| Age * (Range) | 13.64 ± 2.56 (9 – 18) | 14.60 ± 2.67 (10 – 18) | 0.38 |
| Male sex (n,%) | 9 (64%) | 5 (50%) | 0.48 |
| Weight* | 45.78 ± 14.75 | 44.93 ± 11.59 | 0.38 |
| Height * | 151 ± 16 | 150 ± 3.64 | 0.81 |
| Initial FVC%* | 79.14 ± 18.89 % | 82.7 ± 22.57% | 0.67 |
| Initial FEV1%* | 52.42 ± 17.39% | 58.5 ± 23.80% | 0.47 |
| Initial FEV1/FVC * | 56.02 ± 8.45 | 59.53 ± 11.64 | 0.39 |
| Initial FEF 25-75* | 24.07 ± 16.40% | 29.9 ± 22.76% | 0.47 |
| Initial TLC%* | 112.64 ± 12.67% | 114.12 ± 19.46% | 0.83 |
| Initial RV%* | 215.5 ± 51.42% | 198.5 ± 70.46% | 0.52 |
| Initial sGaw | 0.054 ± 0.02 | 0.066 ± 0.02 | 0.38 |
| Note: FVC: Forced Vital Capacity; FEV1: Forced Expiratory Volume in one second; FEV1/FVC (Tiffeneau); FEF25/75 Forced expiratory Flow at 25/75; TLV: Total Lung Capacity; RV: Residual Volume; sGaw: Specific conductance; p ** (< 0.05). | |||
Between the study initiation and end of the study, FVC increased in nine cases in the treatment group, a difference that was significantly higher than expected (p = 0.004) (Table 2). However, when lung function was assessed in control patients, there were no significant differences in FVC, FEV1, TLC, RV, or sGaw between the measurements observed in the treatment group and matched controls (Table 3).
| Table 2: % FVC and FEV1 between study initiation and end of the study. | |||||
| Azithromycin n 12 | p - value* | Placebo n 9 | p - value* | ||
| Relative change % FVC 1 m – 6 m |
> < = |
9 0 3 |
0.004 | 6 2 1 |
0.08 |
| Relative change % EFV1 1 m – 6 m |
> < = |
7 4 1 |
0.20 | 3 3 3 |
0.85 |
| n 12 | n 6 | ||||
| Relative change % TLC 1 m – 6 m |
> < = |
9 2 1 |
0.07 | 3 3 |
0.40 |
| Relative change % RV 1 m – 6 m |
> < = |
6 6 |
0.93 | 5 1 |
0.07 |
| Note: FVC: Forced Vital Capacity; FEV1: Forced Respiratory in One Second; TLC: Total Lung Capacity; RV: Residual Volume p* (< 0.05) |
|||||
| Table 3: Comparison of lung function between patients in the treatment group and matched controls. | ||||
| Gap in % between 0 and 6 PFT X ± DS | Group 1 (AZT) | Group 0 (Placebo) | Difference 95% CI |
p - value U Mann Whitney |
| Gap in % FVC | 7.14 ± 9.7 | 2.6 ± 5.3 | 4.54 (- 2.5 to 11.5) | 0.37 |
| Difference FEV1 % | 2.14 ± 6.22 | - 0.9 ± 6.33 | 3.04 (- 2.3 to 8.42) | 0.31 |
| Difference TLC% | 3.42 ± 7.83 | 2 ± 8.39 | 1.42 (- 5.72 to 8.58) | 0.44 |
| Difference RV% | - 2.57 ± 46.6 | 8.66 ± 16.4 | -11.23 (-46 to 24) | 0.37 |
| Difference sGaw | - 0.007 ± 0.018 | -0.002 ± 0.04 | 0.001 (-0.024 to 0.027) | 0.70 |
| Note: PFT: Pulmonary Function Test; AZT: Azithromycin; FEV1: Forced Vital Capacity; TLC: Total Lung Capacity; RV: Residual Volume; sGaw: Specific conductance | ||||
In each group, three exacerbations were observed, with no significant difference between the two groups.
The results of the quality of life questionnaire showed no change in patients using azithromycin compared with controls.
No differences were found between composite HRCT scan scores at the beginning and at the end of the study. In the treatment group, G1, the mean score at baseline was 15 ± 4.9 with a median score of 15.5, while at the end of the study, the mean score was 13.8 ± 2.1 with a median of 14. In the placebo group, G0, the mean score at baseline was 15.6 ± 5.6 with a median of 15.5, while at the end of the study, the mean score was 13.4 ± 2.7 with a median of 14 (Table 4).
| Table 4: Pre- and post-treatment composite HRCT scan scores in the treatment group and in the placebo group. | |||||
| HRCT score | X ± DS | Median | HRCT score | X ± DS | Median |
| At initiation G1 | 15 ± 4.9 | 15,5 | At study end G1 | 13.8 ± 2.1 | 14 |
| At initiation G0 | 15.6 ± 5.6 | 15.5 | At study end G0 | 13.4 ± 2.7 | 14 |
To our knowledge, this is the first double-blind randomized controlled clinical trial to explore whether long-term treatment with azithromycin provides clinical benefits to patients with PIBO. In this study, we found no evidence of effect after 6 months of azithromycin treatment in patients with PIBO. Neither lung function, exacerbation rates, quality of life questionnaire and HRCT scan score differed significantly.
There is evidence that macrolides reduced morbidity and mortality in diffuse panbronchiolitis in the Japanese population [4,5]. Diffuse panbronchiolitis is similar to bronchiolitis obliterans in terms of inflammatory and obstructive components.
Kudoh, et al. divided a population of 498 patients with panbronchiolitis into three groups according to the years they were treated: Group A in which patients received conventional ATB, group B received treatment with specific ATB for Pseudomonas aeruginosa, such as quinolones, and group C that received treatment with erythromycin. The survival rate of patients in group C was significantly higher than that of patients in group A (p < 0.0001) and B (p < 0.0001) [5].
Wolter, et al. [7] evaluated the effect of azithromycin in patients with CF. In a prospective, randomized, placebo-controlled trial with two arms, 60 adult patients with a mean age of 27.9 years received azithromycin or placebo for 3 months. Both FVC and FEV1 remained stable in patients who received treatment while in those who received placebo, it declined by half (-3.62 and -5.73, respectively). In addition, patients in the treatment arm required less IV ATB than controls (p = 0.0016).
Finally, Saiman, et al. [8] conducted a multicenter, randomized, double-blind, placebo-controlled study with two arms enrolling 185 subjects at 23 CF centers in the United States to determine the efficacy of azithromycin in CF patients over 6 years of age.
Patients received azithromycin three times weekly for 6 months. The main outcome was the change in FEV1 and secondary outcomes included pulmonary exacerbations and weight gain. In the group receiving treatment, FEV1 improved 4.4%, while in the placebo group, a drop of 1.8% was found. The improvement was observed 30 days after treatment initiation and lasted until the study ended. Similar to FEV1, FVC also improved in the group receiving macrolides. In the treatment group, a decrease in the number of exacerbations was observed, together with a 47% reduction in hospital admissions and a 39% decrease in the days of IV ATB.
In a multicenter, double-blind, randomized controlled study of 89 indigenous children from Australia and New Zealand aged 1–8 years with bronchiectasis receiving azithromycin or placebo once per week for up to 2 years, Valery, et al. [13] found that those receiving azithromycin had a significantly lower exacerbation rate; however, these patients only had bronchiectasis and not bronchiectasis secondary to BO [14].
Chang, et al. [15] recommend the use of long-term macrolides in patients with bronchiectasis cautiously, and only in children with frequent exacerbations.
Given the inflammatory nature of the bronchial involvement in BO and the similarity to both panbronchiolitis and the endobronchial inflammatory reaction that occurs in CF and bronchiectasis, we hypothesized that macrolides may be useful in treating patients with PIBO and could cause a decrease in the number of respiratory relapses secondary to infections and improve lung function, thereby decreasing morbidity and mortality of these patients [9].
Definitive conclusions cannot be drawn from our study, as it is limited by a relative lack of power to show differences between the two treatment groups. However, no evidence of efficacy of the drug was found, since no difference was observed in the primary outcome or in the pulmonary exacerbations compared to patients treated with placebo or azithromycin.
Explanations other than the lack of statistical power that may clarify why azithromycin did not appear to be clinically effective are, that these patients have a chronic obstruction and that infection may have occurred many years before treatment. Nevertheless, in a report by Pinto, et al. [14] evaluating patients with acute bronchiolitis, no statistically significant changes in the use of azithromycin or in the number of days of hospitalization and oxygen requirement were found.
In addition in our study azithromycin was administered every other day instead of every day. Nevertheless, azithromycin has a volume of body distribution with a bioavailability of approximately 37%, reaching peak plasma serum levels in 2 to 3 hours. Studies in humans have shown markedly higher azithromycin levels in tissues than in plasma with a high concentration in lung tissue and phagocytes.
Dose and dose interval were selected based on the half-life of the drug and the elimination half-life from the tissues, especially lung tissue, which is 2 to 4 days.
The main route of elimination is through the bile, with possible adverse effects in the liver.
Furthermore, prior studies in patients with CF used the same interval as the one proposed in this study. As the drug was not previously used in patients with PIBO, for this study we adopted doses and dose intervals used in this study.
The effect of macrolide on inflammation also differs in races and depends on HLA B54.
Further long-term studies with larger sample sizes are necessary to draw definite conclusions on the use of azithromycin for the treatment of PIBO. Nevertheless, until further evidence is available we believe that azithromycin should not be used in the treatment of PIBO.
PIBO is a considerable problem in our country. There is a need for further studies that would allow us to find a better treatment for the condition and rule out others, such as azithromycin, that do not seem to be efficacious and increase management costs.
We would like to thank Patricia Murtagh MD † and Manuela DicembrinoMD for the review and comments and Jeeneke Ddeurloog for the English review.
Ethical considerations
This study was performed in accordance with the Declaration of Helsinki. This human study was approved by the Hospital de Pediatria “Juan P. Garrahan” Ethics Committee. The study’s clinical trial registration number is NCT05299567 - registered with NCT.
All parents, guardians, or next of kin provided written informed consent for the minors to participate in this study.
Author contributions
CC: Design, data analysis and interpretation, critical review of the content, manuscript writing, final approval of manuscript, and responsible for answering any questions.
SM: Design, data analysis, and interpretation, critical review of the content, and final approval of manuscript.
PC: Data collection and analysis, critical review of manuscript content, and final approval of manuscript.
AV: Data collection, critical review of manuscript content, and final approval of manuscript.
LB: Data collection, critical review of manuscript content, and final approval of manuscript.
BG: Data collection and final approval of manuscript.
ZB: Design, data analysis, and final approval of manuscript.
LL: Data collection.
GPH: Design, data analysis, and interpretation, critical review of the content, manuscript writing, and final approval of manuscript.
- Grenoville M. Children in the aftermath of acute lower respiratory infections. Module 1 - Pronap 2003.
- Berman S. Epidemiology of acute respiratory infections in children of developing countries. Rev Infect Dis. 1991 May-Jun;13 Suppl 6:S454-62. doi: 10.1093/clinids/13.supplement_6.s454. PMID: 1862276.
- Murtagh P, Cerqueiro C, Halac A, Avila M, Kajon A. Adenovirus type 7h respiratory infections: a report of 29 cases of acute lower respiratory disease. Acta Paediatr. 1993 Jun-Jul;82(6-7):557-61. doi: 10.1111/j.1651-2227.1993.tb12753.x. PMID: 8338989.
- Koyama H, Geddes DM. Erythromycin and diffuse panbronchiolitis. Thorax. 1997 Oct;52(10):915-8. doi: 10.1136/thx.52.10.915. PMID: 9404381; PMCID: PMC1758435.
- Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med. 1998 Jun;157(6 Pt 1):1829-32. doi: 10.1164/ajrccm.157.6.9710075. PMID: 9620913.
- Equi A, Balfour-Lynn IM, Bush A, Rosenthal M. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet. 2002 Sep 28;360(9338):978-84. doi: 10.1016/s0140-6736(02)11081-6. PMID: 12383667.
- Wolter J, Seeney S, Bell S, Bowler S, Masel P, McCormack J. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax. 2002 Mar;57(3):212-6. doi: 10.1136/thorax.57.3.212. PMID: 11867823; PMCID: PMC1746273.
- Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, Coquillette S, Fieberg AY, Accurso FJ, Campbell PW 3rd; Macrolide Study Group. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003 Oct 1;290(13):1749-56. doi: 10.1001/jama.290.13.1749. PMID: 14519709.
- Labro MT. Anti-inflammatory activity of macrolides: a new therapeutic potential? J Antimicrob Chemother. 1998 Mar;41 Suppl B:37-46. doi: 10.1093/jac/41.suppl_2.37. PMID: 9579711.
- Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995 Sep;152(3):1107-36. doi: 10.1164/ajrccm.152.3.7663792. PMID: 7663792.
- Polgar G, Promadhat V. Pulmonary function testing in children: techniques and standards. Philadelphia, WB Saunders C, 1971.
- Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983 Jun;127(6):725-34. doi: 10.1164/arrd.1983.127.6.725. PMID: 6859656.
- Valery PC, Morris PS, Byrnes CA, Grimwood K, Torzillo PJ, Bauert PA, Masters IB, Diaz A, McCallum GB, Mobberley C, Tjhung I, Hare KM, Ware RS, Chang AB. Long-term azithromycin for Indigenous children with non-cystic-fibrosis bronchiectasis or chronic suppurative lung disease (Bronchiectasis Intervention Study): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. 2013 Oct;1(8):610-620. doi: 10.1016/S2213-2600(13)70185-1. Epub 2013 Sep 17. Erratum in: Lancet Respir Med. 2015 Aug;3(8):e29. PMID: 24461664.
- Pinto LA, Pitrez PM, Luisi F, de Mello PP, Gerhardt M, Ferlini R, Barbosa DC, Daros I, Jones MH, Stein RT, Marostica PJ. Azithromycin therapy in hospitalized infants with acute bronchiolitis is not associated with better clinical outcomes: a randomized, double-blinded, and placebo-controlled clinical trial. J Pediatr. 2012 Dec;161(6):1104-8. doi: 10.1016/j.jpeds.2012.05.053. Epub 2012 Jun 28. PMID: 22748516.
- Chang AB, Bush A, Grimwood K. Bronchiectasis in children: diagnosis and treatment. Lancet. 2018 Sep 8;392(10150):866-879. doi: 10.1016/S0140-6736(18)31554-X. Erratum in: Lancet. 2018 Oct 6;392(10154):1196. PMID: 30215382.