More Information
Submitted: March 23, 2023 | Approved: May 11, 2023 | Published: May 12, 2023
How to cite this article: Sarraf S, Singapurwala M, Jain H, Singh R, Julka A. Role of Inflammatory Markers in Predicting Severity in COVID-19 Patients at Tertiary Care Hospital, Ujjain (M.P.). J Pulmonol Respir Res. 2023; 7: 004-009.
DOI: 10.29328/journal.jprr.1001043
Copyright License: © 2023 Sarraf S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: COVID-19; Inflammatory biomarker; Clinical severity
Role of Inflammatory Markers in Predicting Severity in COVID-19 Patients at Tertiary Care Hospital, Ujjain (M.P.)
Shivmohan Sarraf*, Mustafa Singapurwala, Harshit Jain, Ravendra Singh and Arti Julka
R D Gardi Medical College, Jabalpur Medical Sicence University, Madhya Pradesh, India
*Address for Correspondence: Shivmohan Sarraf, R D Gardi Medical College, Jabalpur Medical Sicence University, Madhya Pradesh, India, Email: [email protected]
Originating from China in 2019, the novel Coronavirus Disease 2019 (COVID-19) pandemic had badly affected most of the world causing immense morbidity and mortality. The disease in moderate to severe cases was characterized by intense inflammation leading to ARDS and hypercoagulable states leading to thrombo-embolism and mortality.
Aim: This study aimed to explore the association of inflammatory biomarkers with COVID-19 disease severity in our hospital which became a dedicated COVID hospital during the pandemic.
The World Health Organization (WHO) officially announced COVID-19 as a pandemic disease in March 2020 and was highly contagious and spread by coughing, talking in proximity, or sneezing [1]. It had rapidly spread globally with approximately 66.4 crore cases, 64.40 crore recoveries, and 67.1 lakh deaths (200,000 death within the first four months) till now. In India, there were 4.46 crore cases of which 4.41 crore were recovered and there were 5.30 lakh deaths till now (JHU CSSE COVID-19 Data) [2].
A large proportion of infected COVID-19 cases had very mild symptoms - such as loss of taste or smell, fever, fatigue, and dry cough - or were completely asymptomatic. However, in about 14% of the cases, Acute Respiratory Distress Syndrome (ARDS) can develop which was a potentially fatal condition. ARDS can especially develop in patients predisposed to certain risk factors, such as diabetes mellitus, old age, hypertension, etc. Though COVID-19 manifested primarily as a respiratory condition however it affected the entire body including the cardiovascular, gastrointestinal, nervous, and hematopoietic systems; This can be due to the angiotensin-converting enzyme 2 (ACE2) receptors being abundantly present in many organs of the body [3].
COVID-19 represents a spectrum of clinical severity ranging from asymptomatic to critical pneumonia, acute respiratory distress syndrome (ARDS) and even death.
Therefore, full monitoring of the severity of COVID-19 and effective early intervention were the fundamental measures for reducing disease severity and thus mortality. Inflammatory responses triggered by rapid viral replication of SARS-CoV-2 and cellular destruction were able to recruit macrophages, and monocytes and induce the release of cytokines and chemokines. These cytokines and chemokines then attract immune cells and activate immune responses, leading to cytokine storms and aggravations [4]. Inflammatory markers such as serum ferritin, erythrocyte sedimentation rate (ESR), Neutrophil Lymphocyte ratio (NLR), C-reactive protein (CRP), LDH, D-Dimer and interleukin-6 (IL-6), S. Albumin and Procalcitonin have been reported to be significantly elevated and associated with the high risks of the development of severe COVID-19 [5].
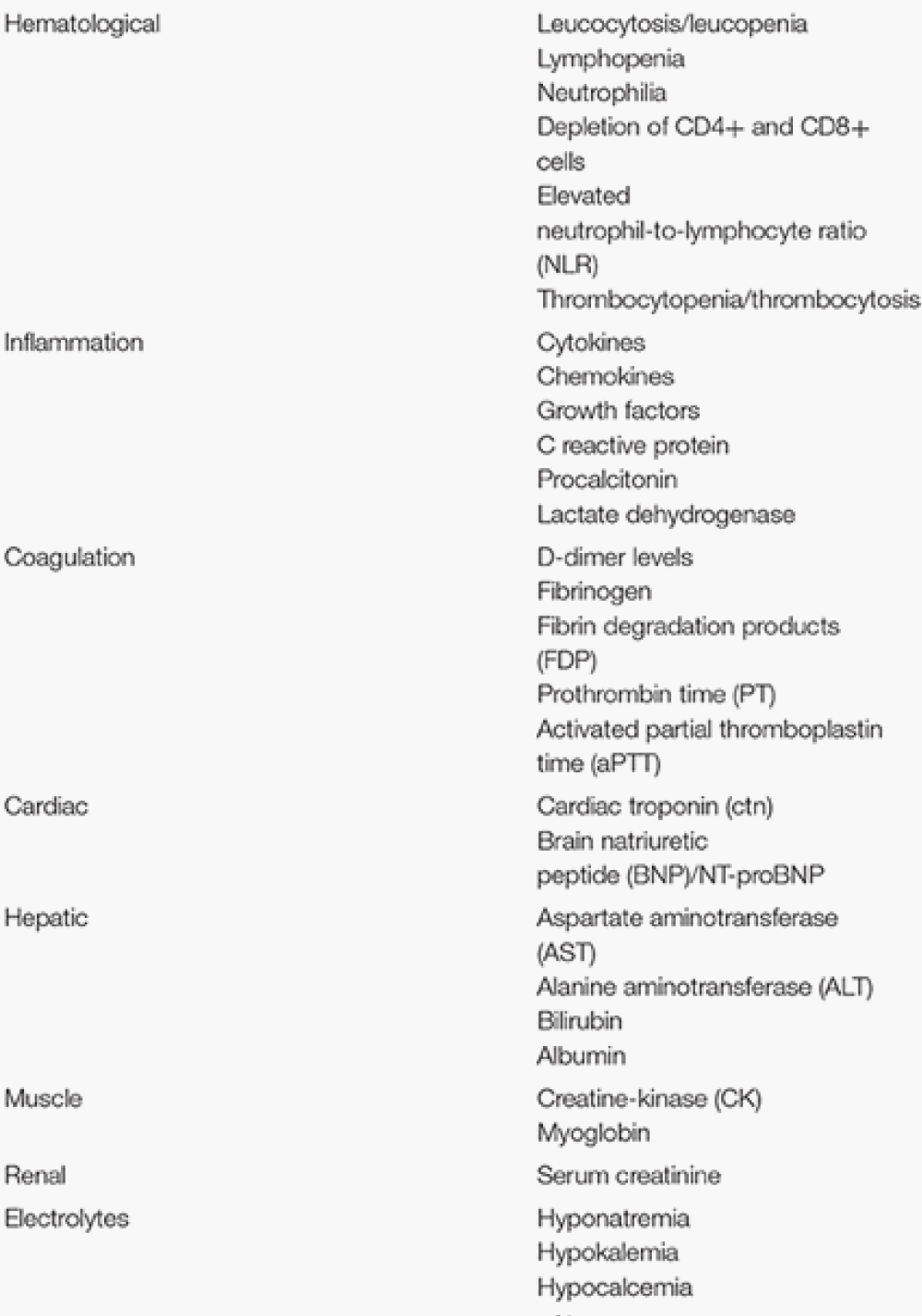
List of various biomarkers in COVID-19 classified according to organ/system involved [6] (Image 1).
Image 1:
The following biomarkers have been identified: hematological (lymphocyte count, neutrophil count, neutrophil-lymphocyte ratio (NLR)), inflammatory (C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), procalcitonin (PCT)), immunological (interleukin (IL)-6 and biochemical (D-dimer, troponin, creatine kinase (CK), aspartate aminotransferase (AST)), especially those related to coagulation cascades in disseminated intravascular coagulation (DIC) and acute respiratory distress syndrome (ARDS).
The role of various Biomarkers in COVID-19 can be useful in the following ways:
(i) Early suspicion of disease
(ii) Confirmation and classification of disease severity
(iii) Framing hospital admission criteria
(iv) Identification of high-risk cohort
(v) Framing ICU admission criteria
(vi) Rationalizing therapies
(vii) Assessing response to therapies and predicting the outcome
(viii) Framing criteria for discharge from the ICU and/or the hospital
So strong knowledge of pathophysiology is a must for the initial identification of candidate biomarkers for understanding what the virus does to the body and how the body reacts to it. In Severe COVID-19 and other critical diseases, there is common inflammatory pathophysiology.
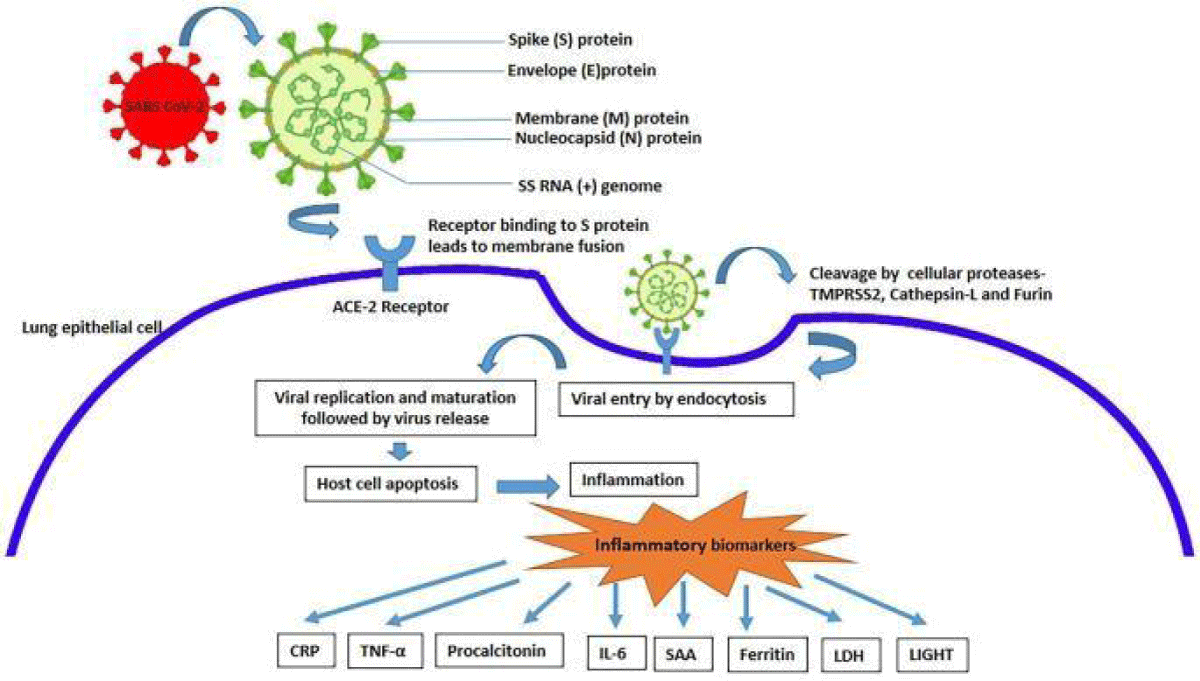
Mechanism of activation of various inflammatory biomarkers in COVID-19 (Image 2).
Image 2:
Biomarkers in COVID-19 is useful for early diagnosis of disease, confirming and classifying disease severity, identifying high-risk severity criteria, deciding admission criteria for ICU requirement, rationalizing therapeutic advances, assessing the response to various therapies, predicting disease outcome, and framing criteria for discharge [6].
The identification of effective laboratory biomarkers able to classify patients based on their risk is for management. It has been found in various studies, for the role of systemic vasculitis and cytokine-mediated coagulation disorders as the principal actors of multi-organ failure in patients with severe COVID-19 complications.
Our medical college hospital during the pandemic became a dedicated COVID tertiary care centre (DCH) and patients were admitted with various clinical manifestations so this study was carried out to evaluate clinical, and radiological severity and its association with different inflammatory markers.
The present observational study was conducted at the Department of Respiratory Medicine, R D Gardi Medical College and C R Gardi Hospital, Ujjain, MP, India. The study protocol was approved by the Institutional Ethics Committee. Our study was carried out on 125 patients admitted to a dedicated COVID hospital. The patients were classified into three groups mild, moderate, and severe COVID- 19 cases on the basis of oxygen saturation on room air measured by pulse oximeter probe and CT severity Score of HRCT Thorax. The disease severity was compared with mean levels of the inflammatory biomarkers when the patient presented to the hospital. The other parameter i.e., age, gender, symptom, comorbidities, addiction, hospital stay, etc. association with disease severity was also compared. The biostatistical test was applied.
Statistical analysis
Statistics analyses were performed using SPSS 21.0 software. Categorical variables were displayed as frequency and percentage while continuous variables were expressed as mean and Standard Deviation (SD). Between groups, a comparison of categorical variables was done using the Chi-square test. An Independent t-test was used for intergroup comparisons of continuous variables. The p - value of < 0.05 was considered statistically significant. The ROC curve analysis was conducted to determine the optimal cut-off of parameters for identifying severe COVID-19 cases. An Area under the Curve (AUC) value of 0.9-1.0 signified a perfect biomarker with excellent accuracy, 0.8-0.9 was very good, 0.6-0.7 was sufficient and a value of 0.5 signified it was no better than what would be expected by chance. The optimal cut-off value was the value that had the highest combined sensitivity and specificity.
In our study out of 125 cases, mild disease was seen in 11(8.8%) patients, moderate in 29(23.2%), and severe in 85(68.0%) patients (Table 1).
| Table 1: Characteristics of study subjects. | |||
| Frequency | Percent | ||
| Age Groups | < = 30 Years | 10 | 8 |
| 31 - 40 Years | 8 | 6.4 | |
| 41 - 50 Years | 16 | 12.8 | |
| 51 - 60 Years | 31 | 24.8 | |
| 61 - 70 Years | 31 | 24.8 | |
| > 70 Years | 29 | 23.2 | |
| Gender | Male | 90 | 72 |
| Female | 35 | 28 | |
| Symptoms | Fever | 103 | 82.40% |
| SOB | 118 | 94.40% | |
| Cough | 98 | 78.40% | |
| Weakness | 119 | 95.20% | |
| Myalgia | 31 | 24.80% | |
| Loss of smell | 89 | 71.20% | |
| Loss of taste | 82 | 65.60% | |
| Sore Throat | 82 | 65.60% | |
| Diarrhoea | 62 | 49.60% | |
| Vomiting | 11 | 8.80% | |
| Loss of Appetite | 94 | 75.20% | |
| Comorbidities | DM(Type2) | 71 | 56.80% |
| HTN | 65 | 52.00% | |
| Thyroid | 27 | 21.60% | |
| COPD/Asthma | 65 | 52.00% | |
| CAD | 42 | 33.60% | |
| T.B | 7 | 5.60% | |
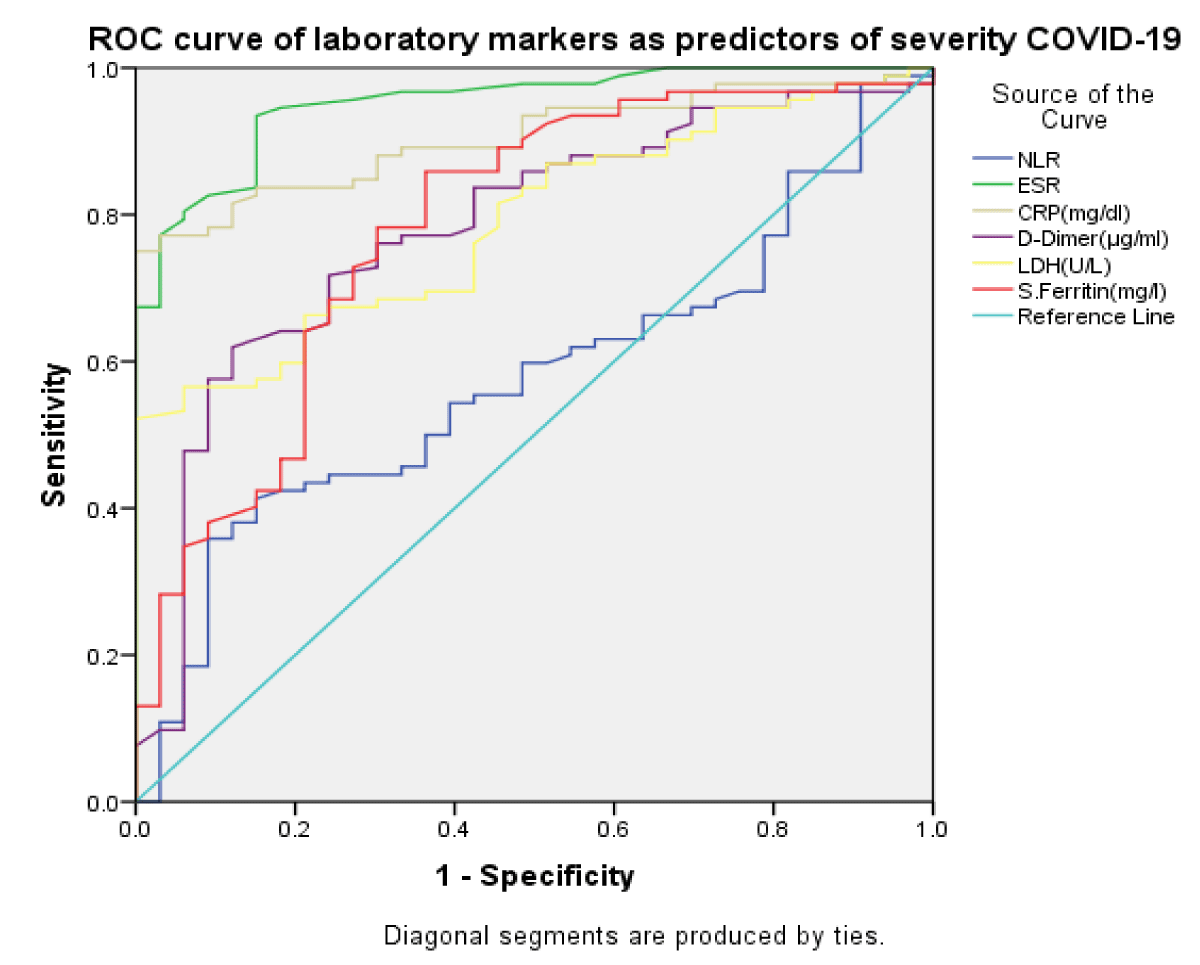
Out of 125 COVID-19 cases, 90(72.0%) were males and 35 (28.0%) were females. In our study, the majority of cases i.e., 62(49.6%) were in the 51-70 age group, 29(23.2%) of the patients were above the age of 70 years, and the rest 34 16(29.2%) cases in 30-50 years age group, In our study showed a mean age of COVID-19 cases of 59.16±16.48 years. The median age was 60 years, the minimum age was 15 years and the maximum age was 92 years. In our study the most common symptom was weakness 119(95.20%), which was followed by SOB (breathlessness) 118(94.4%), fever 103(82.4%), cough 98(78.4%), loss of appetite 94(75.2%), loss of smell 89(71.2%), loss of taste 82(65.6%), sore throat 82(65.6%), diarrhoea 62(49.6%), myalgia 31(24.8%) and vomiting 11(8.8%). The most common comorbidity was DM which was observed in 71(56.8%) of the patients, hypertension in 65(52.0%) and COPD/ Asthma in 65(52.0%), CAD in 42(33.6%) and Active TB in 7(5.6%) of the patients. Out of 125 patients, 97(77.6%) patients had raised NLR, 123(98.4%) had raised CRP, 106(84.8%) had raised D –Dimer, 92(73.6%) had raised LDH, 80(64.0%) raised S. Ferritin and 118(94.4%) had low S. Albumin. In our study, out of 125 cases, the majority of cases 45(36.0%) had 11-15 days hospital stay, 37(29.6%) had 16–20-day hospital stays, 25(20.0%) had less than 10 days hospital stay, 18(14.4%) had more than 20 days hospital stay. Out of 125 cases, 56(44.8%) cases were smokers, 41(32.8%) were alcoholic and 37(29.6%) were tobacco chewer in relation to oxygen requirement, 84(67.2%) cases required ventilator support, 12(9.6%) required NRBM, 9(7.2%) required Bipap, 6(4.8%) put on NP, 6(4.8%) put on FM, 3(2.4%) required HFO and 5(4.0%) cases were on room air Figure 1.
Figure 1: ROC curve with sensitivity and specificity of biomarkers.
The mean value of Hb(g/dl) in the mild, moderate, and severe cases was 12,71,11.44,9.80 respectively which was shown in above Table 2 and haemoglobin levels were found to be significantly lower in patients with severe COVID-19 as opposed to mild to moderate cases. In a meta-analysis by Taneri, et al. [7], haemoglobin levels were found to be significantly lower in patients with severe COVID-19 as opposed to mild to moderate cases [7]. A similar study done by Henry, et al. [8] also showed similar results. The results of our study were also concurring with the results of the above-mentioned studies. Although these mechanisms have been proposed wherein the COVID-19 infection itself leads to a state of anaemia, these are to be substantiated with further evidence.
In our study, a significant association was observed between raised NLR and severity of disease with p < 0.05. The mean value of NLR in mild, moderate, and severe cases was 5.14,7.57,9.09 respectively which was shown in Table 2. The percentage of raised NLR ratio significantly increased from mild to severe cases 54.5%, 89.7%, and 76.5% respectively. NLR showed a good sensitivity of 62% and specificity of 46% respectively with a cut-off of 5.7 in predicting the severity of COVID-19. NLR showed a good sensitivity of 70% and specificity of 58% with a cut-off of 8.8 in predicting the outcome of COVID-19. Henry BM, et al. [8] and Feng X, et al. (2020) NLR is an emerging hematologic marker for the severity of COVID-19 [8]. It is readily available since a complete blood count is a routine workup for patients with suspected infection. An increase in NLR is thought to be caused mainly by increased neutrophil count and decreased lymphocyte count. This points to a hyper-inflammatory state combined with damage to lymphocytes essential for eliminating virus-infected cells. In our study, the mean value of CRP (mg/dl) in mild, moderate, and severe cases was 43.29,320.5,1865.13 respectively which was shown in Table 2. The mean CRP (p < 0.05) was significantly higher among severe cases as compared to moderate and mild cases. Mean CRP (p < 0.05) was significantly higher among death cases as compared to cured cases.
The receiver operator curve analysis of CRP showed a good sensitivity of 88% and specificity of 70% respectively with a cut-off of 80.5 in predicting the severity of COVID-19. CRP, the sensitivity of 88% and specificity of 68%, and 64% respectively with a cut-off of 205 in predicting the outcome of COVID-19.
Cut-off levels have been reported but are not standardized and vary in different settings. CRP is a good marker for acute-phase inflammation and its production in the liver is induced by IL-6, thus considered one of the best surrogates for IL-6 [Henry BM, et al. [8], Herold T, et al. (2020)]. Wang, et al. (n = 209) showed that CRP > 2.69 mg/dL is predictive of aggravation of non-severe COVID-19 patients with 81.3% sensitivity and 79.3% specificity. Wang D, et al. [9]. In the study of Liu, et al. (n = 140), the CRP optimal cut-off at 4.18 mg/dL is predictive of severity with 88.89% sensitivity and 72.73% specificity Liu F, et al. (2020). A similar study by Herold, et al. (n = 89) finds the optimal cut-off for CRP > 3.25 mg/dL at presentation but aimed to determine cut-off values predictive of the need for ICU admission and mechanical ventilation Herold T, et al. (2020). However, the population size has been one of the limitations of the above-mentioned studies. This study shows that a lower cut-off level for CRP is owed to the significantly higher number of patients included.
In our study, the mean value of D-Dimer(mcg/ml) in mild, moderate, and severe cases was 0.90,2.60,3.81 5.14,7.57,9.09 respectively which was shown in Table 2. In our study mean D-Dimmer (p < 0.05) was significantly higher among severe cases as compared to moderate and mild cases. Mean D-Dimmer (p < 0.05) was significantly higher among death cases as compared to cured cases. The receiver operator curve analysis of D-Dimer showed a good sensitivity of 71% and specificity of 76% with a cut-off of 1.23 in predicting the severity of COVID-19. D-Dimer showed a good sensitivity of 68% and specificity of 64% with a cut-off of 1.45 in predicting the outcome of COVID-19. Zhang, et al. (2020) reported an optimum D-dimer with the cutoff value of 2.0 mg/ml within 24 h of hospital admission to predict in-hospital mortality, with a sensitivity of 92.3% and a specificity of 83.3% [9]. Yao, et al. reported D-dimer levels of > 2.14 mg/ml on admission as a predictor of mortality, with a sensitivity of 88.2% and a specificity of 71.3%. Mamta Soni, et al. [10]. Showed that D-dimer levels on admission were a predictor of mortality [10]. D-dimer value of > 1.44 mg/ml within 24 h of hospital admission had a sensitivity of 60.5% and a specificity of 74.0%.
| Table 2: Mean values of inflammatory markers with severity of disease. | |||||||
| Biomarkers | Severity of disease | p | |||||
| Mild (0 - 7) |
Moderate (8 - 17) |
Severe (17 - 24) |
|||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Hb(g/dl) | 12.718 | 2.270 | 11.441 | 2.107 | 9.809 | 1.927 | 0.000 |
| NLR | 5.14 | 3.63 | 7.57 | 4.75 | 9.09 | 5.55 | 0.043 |
| ESR | 17.64 | 2.01 | 57.28 | 20.66 | 82.99 | 20.07 | 0.000 |
| S.Albumin(g/dl) | 2.97 | .76 | 2.30 | .63 | 2.19 | .59 | 0.001 |
| CRP (mg/dl) | 43.29 | 27.35 | 320.59 | 499.48 | 1865.13 | 3733.41 | 0.027 |
| D-Dimer (µg/ml) |
0.90 | 0.58 | 2.60 | 4.44 | 3.81 | 4.29 | 0.04 |
| LDH(U/L) | 352.17 | 150.23 | 528.78 | 618.16 | 936.22 | 1205.33 | 0.04 |
| S. Ferritin(mg/l) | 206.82 | 133.79 | 456.90 | 370.23 | 612.80 | 434.56 | 0.004 |
In our study the mean value of LDH(U/L)) in the mild, moderate, and severe cases was 352.17,618.16,936.22 respectively which was shown in above Table 2, and the inflammatory marker LDH increasing with the increased disease severity. The mean LDH level (p < 0.05) was significantly higher among severe cases as compared to moderate and mild cases.
Inflammatory markers mean LDH level (p < 0.05) was significantly higher among death cases as compared to cured cases.
In our study, the receiver operator curve analysis of LDH showed good sensitivity of 72% and specificity of 58% with a cut-off of 430 in predicting the severity of COVID-19. LDH showed a good sensitivity of 68% and specificity of 62% with a cut-off of 503 in predicting the outcome of COVID-19.
Zhang Z, et al. (2020) give an LDH level > 644.85 U/L predicts COVID-19 severity in this study. The level of LDH was elevated in both groups (normal range 140-280U/L) (Holmes RS, et al. 2020), representing cell death (apoptosis), these values correlated well with lymphopenia and IL-2Ra, and the results were concomitant with the recently published data by Zhang Z meta-analysis [10]. Many studies did not include LDH in their investigations. But a meta-analysis showed that LDH is significantly higher in the severe group compared to the non-severe group.
In our study, the mean value of S. Ferritin (mg/l) in the mild, moderate, and severe cases was 206.82,456.9,612.80 respectively which was shown in Table 2 and inflammatory marker mean S. Ferritin level (p < 0.05) was significantly higher among severe cases as compared to moderate and mild cases. The mean S. Ferritin level (p < 0.05) was significantly higher among death cases as compared to cured cases.
In our study, the receiver operator curve analysis of S. Ferritin showed a good sensitivity of 75% and specificity of 70% with a cut-off of 330 in predicting the severity of COVID-19 which was shown in Table 2. S. Ferritin showed a good sensitivity of 70% and specificity of 65% with a cut-off of 451 in predicting the outcome of COVID-19.
Zeng F, et al. [11] a ferritin level > 621.4 ng/mL also predicts COVID-19 severity in this study but with relatively lower sensitivity and specificity compared with CRP and LDH. This could be attributed to the tendency of ferritin to increase during inflammation, as well as in liver disease and malignancy. However, the result is consistent with the meta-analysis that shows ferritin as a strong discriminator for severe disease Henry BM, et al. [8]. Another meta-analysis reports that ferritin levels were consistently higher in the severe versus non-severe group and have a potential role in monitoring disease progression Zeng F, et al. [11].
We investigated the association between key inflammatory markers and a wide range of clinical characteristics and laboratory variables as well as categorized disease severity in the 125 COVID-19 patients. Our results showed elevated CRP, D-Dimer, serum ferritin, and serum LDH have the highest level of significance (p < 0.0001) along with elevated NLR, low Hb, and low albumin with more severe cases including patients admitted to the ICU unit. Similarly, elevated neutrophils count, in addition to CRP, D-Dimer, serum ferritin as well as LDH were strongly associated with poor outcomes and more death compared to patients who were alive. This finding goes with recent reports that showed high levels of CRP, D-dimer, LDH, and ferritin are associated with a more severe clinical course and can predict poor prognosis. Since the inflammation was the reason for ARDS and cardiac complications associated with the COVID-19.
This study suggested that CRP, NLR, LDH, S ferritin, S. Albumin, and D-dimer can be helpful in monitoring COVID-19 patients for early identification of severe cases which can be useful to reduce or prevent progression toward critical stage and mortality by using early clinical and therapeutical interventions based upon these biomarkers. The inflammatory markers were means for diagnosis, evaluation of severity and response to therapy, and for prediction of prognosis.
Limitations
In the present study, though a small number of patients were included using a convenient sampling method which may not be fully representative of the overall COVID-19 disease status. The results of our study need to be considered in the context of potential limitations.
Firstly, this is a single-center and observational study. Large prospective studies, over an extended period, are needed to verify our results.
Secondly, the inflammatory response associated with NLR, ESR, CRP, LDH, and S. Ferritin shows promise in ROC AUC analysis to predict severe disease and outcome of COVID-19 cases obtained on the basis of a small sample size. Inflammatory markers i.e., Procalcitonin, and IL6 which are important markers for COVID 19 were not available in our center.
- WHO Director-General’s opening remarks at the media briefing on COVID19 -March 2020
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533-534. doi: 10.1016/S1473-3099(20)30120-1. Epub 2020 Feb 19. Erratum in: Lancet Infect Dis. 2020 Sep;20(9):e215. PMID: 32087114; PMCID: PMC7159018.
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Apr;8(4):420-422. doi: 10.1016/S2213-2600(20)30076-X. Epub 2020 Feb 18. Erratum in: Lancet Respir Med. 2020 Feb 25;: PMID: 32085846; PMCID: PMC7164771.
- Keam S, Megawati D, Patel SK, Tiwari R, Dhama K, Harapan H. Immunopathology and immunotherapeutic strategies in severe acute respiratory syndrome coronavirus 2 infection. Rev Med Virol. 2020 Sep;30(5):e2123. doi: 10.1002/rmv.2123. Epub 2020 Jul 9. PMID: 32648313; PMCID: PMC7404843.
- Gong J, Dong H, Xia QS, Huang ZY, Wang DK, Zhao Y, Liu WH, Tu SH, Zhang MM, Wang Q, Lu FE. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19: a retrospective study. BMC Infect Dis. 2020 Dec 21;20(1):963. doi: 10.1186/s12879-020-05681-5. PMID: 33349241; PMCID: PMC7750784.
- Samprathi M, Jayashree M. Biomarkers in COVID-19: An Up-To-Date Review. Front Pediatr. 2021 Mar 30;8:607647. doi: 10.3389/fped.2020.607647. PMID: 33859967; PMCID: PMC8042162.
- Taneri PE, Gómez-Ochoa SA, Llanaj E, Raguindin PF, Rojas LZ, Roa-Díaz ZM, Salvador D Jr, Groothof D, Minder B, Kopp-Heim D, Hautz WE, Eisenga MF, Franco OH, Glisic M, Muka T. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol. 2020 Aug;35(8):763-773. doi: 10.1007/s10654-020-00678-5. Epub 2020 Aug 20. PMID: 32816244; PMCID: PMC7438401.
- Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020 Jun 25;58(7):1021-1028. doi: 10.1515/cclm-2020-0369. PMID: 32286245.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 Mar 17;323(11):1061-1069. doi: 10.1001/jama.2020.1585. Erratum in: JAMA. 2021 Mar 16;325(11):1113. PMID: 32031570; PMCID: PMC7042881.
- Soni M, Gopalakrishnan R, Vaishya R, Prabu P. D-dimer level is a useful predictor for mortality in patients with COVID-19: Analysis of 483 cases. Diabetes Metab Syndr. 2020 Nov-Dec;14(6):2245-2249. doi: 10.1016/j.dsx.2020.11.007. Epub 2020 Nov 17. PMID: 33395786; PMCID: PMC7670909.
- Zeng F, Huang Y, Guo Y, Yin M, Chen X, Xiao L, Deng G. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int J Infect Dis. 2020 Jul;96:467-474. doi: 10.1016/j.ijid.2020.05.055. Epub 2020 May 18. PMID: 32425643; PMCID: PMC7233226.