More Information
Submitted: December 05, 2022 | Approved: December 22, 2022 | Published: December 23, 2022
How to cite this article: Cheikhrouhou T, Dhaou MB, Elleuch A, Hbaieb M, Zouari M, et al. The thoracoscopic approach in the management of parapneumonic pleural effusion in children. J Pulmonol Respir Res. 2022; 6: 025-029.
DOI: 10.29328/journal.jprr.1001041
Copyright License: © 2022 Cheikhrouhou T, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Parapneumonic pleural effusion; Empyema; Thoracoscopy; Children
Abbreviations: VATS: Video-Assisted Thoracoscopic Surgery; US: Ultrasound; CT: Computed Tomography
The thoracoscopic approach in the management of parapneumonic pleural effusion in children
Taycir Cheikhrouhou1,3* , Mahdi Ben Dhaou1,3, Amal Elleuch2,3, Manar Hbaieb1,3, Mohamed Zouari1,3, Mahfoudh Abdelmajid2,3 and Riadh Mhiri1,3
, Mahdi Ben Dhaou1,3, Amal Elleuch2,3, Manar Hbaieb1,3, Mohamed Zouari1,3, Mahfoudh Abdelmajid2,3 and Riadh Mhiri1,3
1Department of Pediatric Surgery, Hedi Chaker Hospital, University of Sfax, Sfax, Tunisia
2Department of Pediatrics, Habib Bourguiba Hospital, University of Sfax, Sfax, Tunisia
3University of Medicine of Sfax, University of Sfax, Sfax, Tunisia
*Address for Correspondence: Taycir Cheikhrouhou, Department of Pediatric Surgery, Hedi Chaker Hospital, University of Sfax, Sfax, Tunisia, Email: [email protected]
Background: Parapneumonic pleural effusion is a relatively common entity and continues to be a major cause of morbidity in children. However, managing this disease is still a matter of controversy between surgical and non-surgical options. With the advancement of mini-invasive surgery, video-assisted thoracoscopic surgery (VATS) has become a mainstay in the treatment of parapneumonic effusion in children. This study aimed to evaluate the clinical characteristics and pathological features of parapneumonic pleural effusion in children and to explore the feasibility and safety of the thoracoscopic approach in the pediatric population.
Methods: The clinical data of all patients who underwent VATS for parapneumonic effusion between 2007 and 2021 were analyzed retrospectively. Factors that were documented included demographic criteria, clinical manifestations, preoperative examinations, therapeutic procedures, intraoperative findings, postoperative complications, and outcomes.
Results: Totally, 35 patients with a mean age of 5.14 ± 3.9 years were operated on thoracoscopically. The mean duration of evolution before VATS was 9 days ± 4. All children were hospitalized in a Pediatric Continuing Care Unit. Antibiotic therapy was administrated in combination in all cases. Corticosteroid therapy was used in 2 patients. Thoracentesis was performed in 6 patients. Thoracostomy tube drainage was placed before surgery in 11 patients. The average duration of drainage before VATS was 6 days ± 4. VATS decortication and/or debridement was indicated as second-line in 23 patients. The average duration of the surgery was 51 minutes (20 min - 115 min). There is no conversion to open surgery and no intraoperative procedure-dependent complication. 4 children have early complications after the VATS and one patient had a late postoperative complication. There were no deaths during the hospital stay or follow-up.
Conclusion: In skilled hands, VATS is safe, feasible, and effective in the management of parapneumonic pleural effusion in children with excellent outcomes.
Parapneumonic effusion is defined as pleural effusion associated with lung infection [1-4]. These effusions result from the spread of inflammation and infection to the pleura. The pleura becomes inflamed early in the course of parapneumonic effusion, and subsequent leakage of proteins, fluid, and leukocytes into the pleural space forms the effusion. The pleural effusion is usually sterile and has a low leukocyte count when it forms. Bacteria infiltrate the fluid over time, resulting in empyema, which is defined as the presence of grossly purulent fluid in the pleural cavity [5]. It is associated with significant morbidity, including prolonged hospitalization and frequent, and sometimes repeated, invasive acts [5-7]. Treatment is still a matter of controversy between surgical and non-surgical options [2,3,6,8]. Therapeutic options include antibiotics, thoracentesis, thoracostomy tube drainage, fibrinolysis, video-assisted thoracoscopic surgery (VATS), and thoracotomy [2].
With the advancement of mini-invasive surgery, VATS has become a mainstay in the treatment of parapneumonic effusion in children, shortening hospitalization and decreasing the need for more invasive surgery. The timing of the thoracoscopy is critical [9]. However, few studies have evaluated the safety and feasibility of the thoracoscopic approach in the management of parapneumonic pleural effusion in children [2].
We aimed to present our experiences with parapneumonic effusion management in children and to evaluate the feasibility, safety and radicality of the thoracoscopic approach.
We conducted a retrospective study of the charts of all patients who underwent VATS for parapneumonic effusion between 2007 and 2021. Patients with the obvious non-infectious cause or insufficient data in the hospital chart or lost during follow-up were excluded from the analysis. Ethical consent was obtained from the local board.
Any patient between the ages of 0 and 15 with evidence of community-acquired bacterial pneumonia and an associated parapneumonic effusion was eligible to enroll in the study.
Qualification for thoracoscopic intervention was performed in the pediatric-surgical team based on the history, clinical symptoms, and imaging tests according to the established pattern.
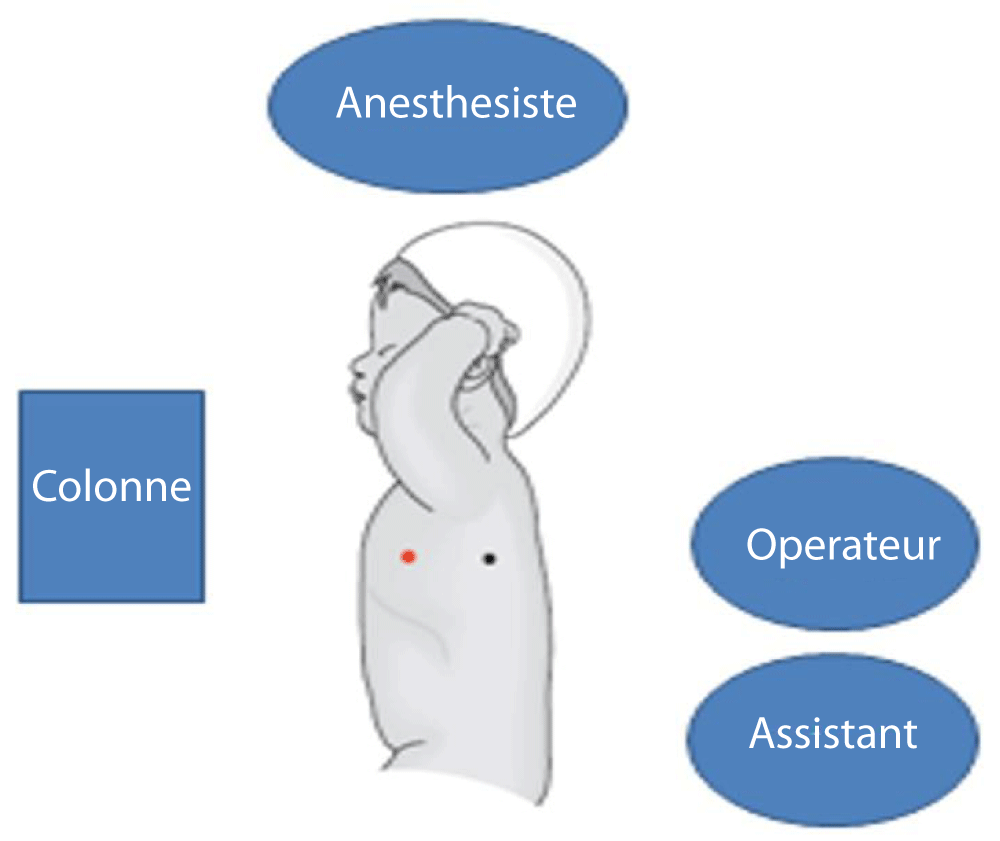
The technique of the procedure: The thoracoscopy procedure was performed under general anesthesia with endotracheal intubation with a single lumen endotracheal tube. The patient was placed in a lateral decubitus position with pronounced intercostal space (Figure 1).
Figure 1: Patient position, crew and equipment.
The place of insertion of the first 5 mm or 10 mm optical trocar was at the point of intersection of the middle axillary line and the fifth or sixth intercostal space. The 5 mm working trocars (one or two) were located under visual control and depending on the anatomical conditions prevailing in the inflamed pleural cavity.
In all patients, the bacteriological examination was collected, pleural biopsies were performed and then we performed pneumolysis or pleurolysis, a toilet of the pleural cavity with physiological serum, and finally double drainage of the pleural cavity (the first drain in the posterior and upper direction and the second drain in the basal direction).
We recorded the data of demographic characteristics, clinical manifestations, details of preoperative examination, the therapeutic procedure performed, intraoperative findings, short-term and midterm complications, and outcomes. The follow-up period ranged from 6 months to 9 years.
All data were processed using descriptive statistical procedures for calculating means, frequencies, and percentages.
General clinical data
A total of 35 patients (15 boys and 20 girls, sex-ratio = 0.75) were identified with parapneumonic effusions and treated by VATS, between 2007 and 2021 at the department of pediatric surgery at Hedi Chaker Hospital. The patient’s age ranged from 6 months to 14 years (mean 5.14 ± 3.9 years). Among them, 54.3% of VATSs were made in winter (28.6% in February). A history of infectious pneumonitis was found in 40% of cases. The mean duration of evolution before VATS was 9 days ± 4 (4 to 21 days). All children had a fever, and 14 of them (40% of the cases) persisted for more than a week. Respiratory signs were found in 40% of the cases. At physical examination, all children exhibited abolition of vesicular murmuring on the effusion side and alteration of the general condition in 71.5% of the cases.
Preoperative complementary investigation
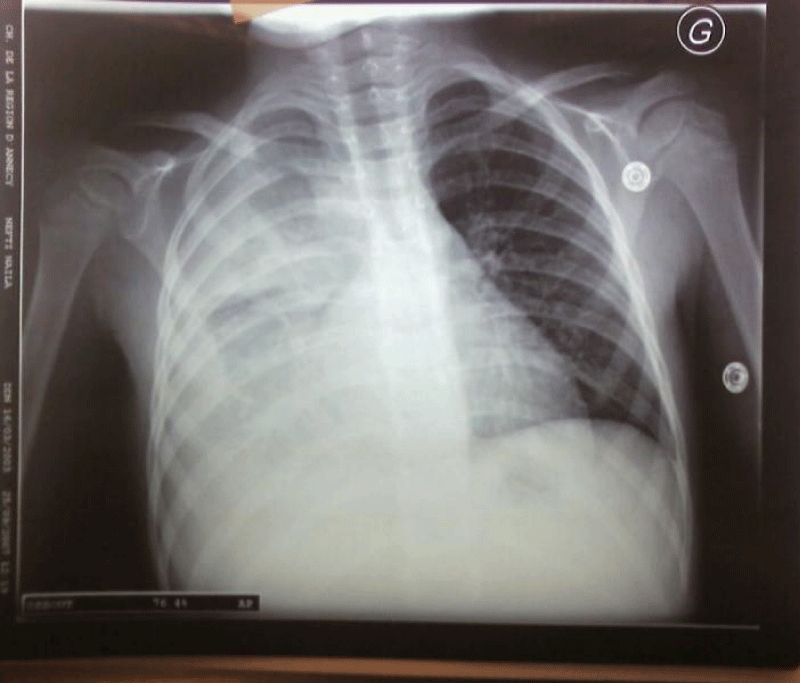
The results of blood investigations showed a persistent inflammatory biological syndrome in all patients. All children had chest radiographs performed and all were abnormal: 22 children (62.9%) had right-sided involvement (Figure 2).
Figure 2: Chest X-ray showing right pleural effusion.
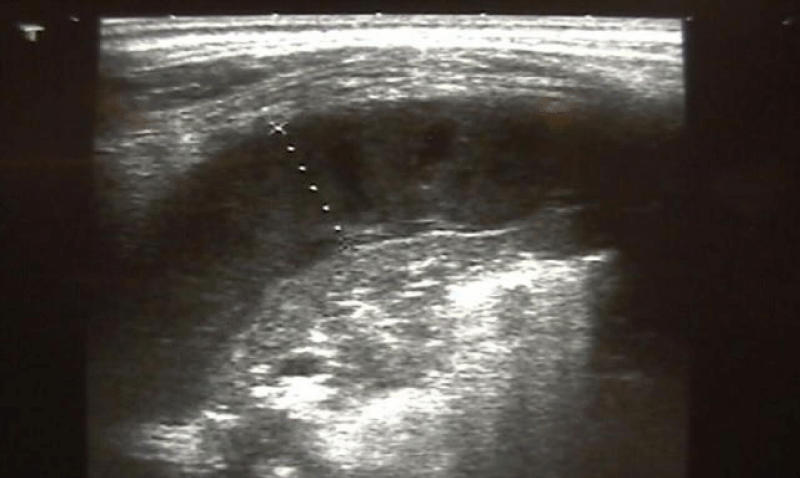
Chest ultrasound (US) was performed in all children and showed a hemi-circumferential effusion in 3 cases (8.7%), thickness between 2 cm and 5 cm in 17 cases (48.6%), and signs of gravity: pneumothorax in 5 cases (14.3%), mediastinal deviation in 6 cases (17.1%), pleural partitioning in 30 cases (85.7%) and pleural abscesses in 2 cases (5.8%). (Figure 3).
Figure 3: Chest ultrasound showing the volume of effusion.
Computed tomography (CT) was performed in 22 patients (62.9%). The results of CT exploration are summarized in Table 1.
| Table 1: Results of CT exploration. | |
| Results of CT exploration | Number of patients (%) |
| Hemi-circumferential pleural effusion | 6 (27.3%) |
| Bilateral pleural effusion | 2 (9.1%) |
| Thickness >3 cm | 10 (45.5%) |
| Signs of severity | |
| - Pneumothorax - Mediastinal deviation - Partitioning - Abscess Pleural - Areas of pulmonary parenchyma necrosis - Atelectasis - Ventilation Disorder - Hepatization of pulmonary parenchyma |
6 (2.3%) 15 (68.2%) 5 (22.7%) 4 (18.2%) 3 (13.6%) 1(4.5%) 5 (22.7%) 2 (9.1%) |
Initial therapeutic treatment before VATS
All children were hospitalized in a Pediatric Continuing Care Unit, Oxygen therapy was performed in 30 cases (85.7%) and only 2 patients (5.8%) had respiratory assistance. Antibiotic therapy was administrated in combination (mean duration = 6 days ± 3.4) in all patients. Corticosteroid therapy was used in 2 patients (5.8%). Thoracentesis was performed in 6 patients (17.1%). Thoracostomy tube drainage was placed before surgery in 11 patients (31.4%) with six positive cultures (4 streptococcus Pneumoniae, 1 staphylococcus aureus, and 1 streptococcus constellatus). The average duration of drainage before VATS was 6 days ± 4. All the children had respiratory physiotherapy but no children received intrapleural fibrinolysis before VATS.
VATS
Of the 35 patients, VATS decortication and/or debridement was indicated as second-line in 23 patients (65.7%). Indications were summarized in Table 2.
| Table 2: Indications for video-assisted thoracoscopic surgery. | |
| Indications for VATS | N (%) |
| The worsening of clinical status with an increase in oxygen requirements | 25 (71.5%) |
| The failure of medical treatment | 23 (65.7%) |
| The appearance of signs of radiological gravity: Volume of effusion, Pneumothorax Mediastinal deviation, Partitioning, Parenchymal necrosis | 31 (88.6%) |
Intraoperative findings
Intraoperatively, the severity of the empyema, the accumulation of fibrinous aggregates forming the pleural empyema, and the coexistence of pulmonary abscesses, parenchymal necrosis, or pyothorax, were assessed (Table 3).
| Table 3: Distribution of patients depending on intraoperative finding | ||
| Intraopérative finding | Number of cases | % |
| Abces | 2 | 5.7 |
| Pleural empyema | 1 | 2.9 |
| Fibro-purulent | 1 | 2.9 |
| Necrosis | 2 | 5.7 |
| Pachypleuritis | 1 | 2.9 |
| Pyothorax | 1 | 2.9 |
| Sero-fibrinous | 27 | 77.1 |
| Total | 35 | 100% |
Outcomes
The average duration of the surgery was 51 minutes (20 min - 115 min). There is no conversion to open surgery and no intraoperative procedure-dependent complication. Extubation was performed immediately after surgery for 24 patients (68.6%) with an average duration of intubation of one day ± 2.5. Respiratory improvement was in less than two days in 25 patients (71.4%) with an average duration of 2.4 days ± 2.4. The mean duration of the febrile state was 1.8 days ± 1.2. A biological improvement was at least on the third day after thoracoscopy in 27 patients (77.1%). Radiological improvement was observed in 24 cases (68.6%) with an average duration of 3.6 ± 4.3 days.
Otherwise, antibiotic therapy was given with an average duration of 10 days ± 4.2 (4-21 days). Corticosteroid therapy was administrated to 14 patients (40%) with an average duration of 24.5 days ± 18.8. Fibrinolysis was used in 8.6% of cases. The mean time of drainage of the pleural cavity after the procedure was 4.2 days ± 4.6 for the first drain and 5.6 days ± 4.4 for the second one. The mean length of hospital stay after the thoracoscopic procedure was 12 days ± 7.8 and there is no recurrence.
4 children have early complications after the VATS, namely, laryngotracheal injury (N = 1), pneumothorax (N = 1), pneumomediastinum associated with pneumothorax (N = 1), and hemothorax (N = 1). One patient had a late postoperative complication: he presented a pneumothorax two weeks after the removal of the two chest drains. There were no deaths during the hospital stay or follow-up.
Parapneumonic pleural effusion is the most frequent complication of bacterial pneumonia in childhood. In some series, up to 30% – 40% of pneumonia requiring hospitalization are complicated by pleural effusion, and 0.6% – 2% progress to empyema [10]. Empyema evolves in three stages: stage 1 is the “exudative” phase with thin and free-flowing, purulent fluid; stage 2 is the “fibrinopurulent” stage with a thickening of the pleural exudate and formation of loculations; stage 3 is the “organized ” stage with fibrosis and scar formation. The incidence of parapneumonic effusion in the pediatric population is increasing worldwide [11]. The reasons for this significant increase are unknown, but possibilities include the increase in bacterial resistance, climatic changes, the introduction of the pneumococcal vaccine, and the prescription of antibiotics from primary care [12].
In our series, we observed a median age of 5.14 years and a slightly higher incidence in females, results similar to those described in the literature [2,13]. This study found that 54.3% of cases were admitted during the winter. This result was consistent with previous reports [14].
As in our series, physical examination, radiography, and thoracic ultrasound were used to establish the diagnosis in other studies. Imaging is crucial in pleural space disease diagnosis and treatment. Chest x-rays, US, and CT scans are the most commonly used methods of exploration. Conventional radiography has a place in diagnostic time and disease monitoring, but its impact on severity assessment is declining [14]. The pulmonary US allows for the emergent diagnosis of pneumothorax (without waiting for a chest x-ray) with a sensitivity greater than 95% versus less than 60% for a chest x-ray and a negative predictive value of 100% [15]. Because of its lower cost, greater availability, portability, discretionary use of sedation, and lack of ionizing radiation, the US has advocated for confirming the presence of an effusion. Some studies have based the treatment of parapneumonic effusions on whether or not adhesions were detected using the US [16]. In our study, pleuro-pulmonary ultrasound was used to look for signs of the severity of pleuro-pneumonitis. According to Berlioz M and al [17], a CT scan is only used to confirm a surgical indication and to assess the condition of the underlying pulmonary parenchyma before surgery. Some authors recommend it away from pleurisy to assess the parenchymal and pleural sequelae [18,19]. In our series, a CT scan was used in 62.9% of the cases; it has been recommended in cases of slow evolution, puncture, drainage failure, or before surgical indication.
Historically, the treatment of parapneumonic effusions was primarily nonoperative (antibiotics and thoracentesis or chest tube drainage). Although antibiotics and thoracostomy tube drainage may be adequate therapy for early (stage 1) parapneumonic effusions, the presence of loculations and fibrinous adhesions frequently limits this therapy’s success [3]. It is frequently difficult to distinguish between stage 1 and stage 2 disease clinically and radiographically. As a result, this primary nonoperative approach frequently leads to extended hospitalizations [2]. Many retrospective case series have suggested that children who fail conventional chest tube therapy improve after thoracotomy or VATS, especially if the procedure is performed early [9]. Based on these reports, many pediatric surgeons believe that primary VATS is a better approach for children with parapneumonic processes [6,9,13]. When compared to a conventional thoracotomy, VATS has the potential for better lung expansion after the removal of pleural debris and exudate, an excellent enlarged view of the pleural space, optimization of chest tube location, and reduction of chest wall trauma [13].
However, the main prognostic factor for the treatment of pleural empyema by thoracoscopy is the interval between diagnosis and surgery. A delay of more than 4 days between diagnosis and surgery was associated with more frequent surgical difficulties, a longer duration of intervention, longer post-operative apyrexia, longer drainage time, longer hospitalization, and more postoperative complications [20]. A retrospective study of children with documented empyema found that VATS performed within 48 hours of diagnosis reduced hospitalization time by an average of four days [21]. In our series, the mean duration of evolution before VATS was 9 days ± 4 (4 to 21 days) and we opted for the VATS as the second treatment in 65.7% of cases.
Because of current delays in the management of purulent pleurisy , most children are seen in the fibro-purulent stage, with a significant risk of conservative treatment failure. As a result, it is critical that we evaluate these children to the best of our ability to optimize the therapeutic option.
The VATS allows for precise positioning of the chest tube, as well as complete pleural fluid discharge and pneumolysis or extreme pulmonary «decortication.» Most importantly, it hastens complete lung expansion, which reduces hospitalization [2,3,6,7,9,13,20]. In our series, the mean persistence duration of the biological inflammatory syndrome was 3 days ± 1.4 with extremes ranging from 2 days to 9 days and this was comparable with the results of other studies. The mean duration of the radiological improvement was 3.6 days ± 4.3 with extremes ranging from 1 day to 7 days and this was comparable with the literature [2].
For long-term results, aesthetic results are satisfactory and complications are rare. The majority of children with parapneumonic effusions often have favorable long-term outcomes. This is especially true with adults, as they generally do not have chronic underlying lung disease [3].
We are aware of the limitations of the study. They are small groups, which undermines the statistical results; the long period of collecting the data could bias the results due to changes in the disease itself and clinical management (antibiotics, increased recognition of the need for surgical management). The study relies on retrospective data and its focus is objective outcomes; thus, the patient perspective is not known.
The current treatment times for purulent parapneumonic pleural effusion are such that the majority of children are seen in the fibro-purulent stage, with a high risk of treatment failure. It appears to be critical, then, to evaluate these children as thoroughly as possible to optimize therapeutic choices. In skilled hands, VATS is safe, feasible, and effective in the management of parapneumonic pleural effusion in children with excellent outcomes in terms of decreased hospitalization time, decreased chest drainage time, rapid recovery, and reduced duration of parenteral antibiotic therapy. Further large studies are required to confirm these findings. Furthermore, the issue of the indication and location of a prospective VATS during the progression of a medically managed parapneumonic pleural effusion persists.
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc. 2006;3(1):75-80. doi: 10.1513/pats.200510-113JH. PMID: 16493154.
- Kurt BA, Winterhalter KM, Connors RH, Betz BW, Winters JW. Therapy of parapneumonic effusions in children: video-assisted thoracoscopic surgery versus conventional thoracostomy drainage. Pediatrics. 2006 Sep;118(3):e547-53. doi: 10.1542/peds.2005-2719. Epub 2006 Aug 14. PMID: 16908618.
- Hendaus MA, Janahi IA. Parapneumonic Effusion in Children: An Up-to-Date Review. Clin Pediatr (Phila). 2016 Jan;55(1):10-8. doi: 10.1177/0009922815589917. Epub 2015 Jun 7. PMID: 26054782.
- Thimmesch M, Mulder A, Lebrun F, Piérart F, Genin C, Loeckx I, Demaret P. Management of parapneumonic pleural effusion in children: Is there a role for corticosteroids when conventional nonsurgical management fails? A single-center 15-year experience. Pediatr Pulmonol. 2022 Jan;57(1):245-252. doi: 10.1002/ppul.25699. Epub 2021 Sep 29. PMID: 34559458.
- Buckingham SC, King MD, Miller ML. Incidence and etiologies of complicated parapneumonic effusions in children, 1996 to 2001. Pediatr Infect Dis J. 2003 Jun;22(6):499-504. doi: 10.1097/01.inf.0000069764.41163.8f. PMID: 12799505.
- Barglik R, Grabowski A, Korlacki W, Pasierbek M, Modrzyk A. Pleural empyema in children - benefits of primary thoracoscopic treatment. Wideochir Inne Tech Maloinwazyjne. 2021 Mar;16(1):264-272. doi: 10.5114/wiitm.2020.97443. Epub 2020 Nov 26. PMID: 33786143; PMCID: PMC7991945.
- Huang JX, Chen Q, Hong SM, Hong JJ, Cao H. Uniportal Thoracoscopic Debridement for Children With Refractory Pleural Empyema: Case Series of 21 Patients. Front Pediatr. 2021 Nov 24;9:777324. doi: 10.3389/fped.2021.777324. PMID: 34900876; PMCID: PMC8652197.
- Krishnan S, Amin N, Dozor AJ, Stringel G. Urokinase in the management of complicated parapneumonic effusions in children. Chest. 1997 Dec;112(6):1579-83. doi: 10.1378/chest.112.6.1579. PMID: 9404757.
- Pappalardo E, Laungani A, Demarche M, Erpicum P. Early thoracoscopy for the management of empyema in children. Acta Chir Belg. 2009 Oct;109(5):602-5. doi: 10.1080/00015458.2009.11680495. PMID: 19994802.
- Martinón-Torres F, Bernaola Iturbe E, Giménez Sánchez F, Baca Cots M, de Juan Martín F, Díez Domingo J, Garcés Sánchez M, Gómez Campderá JA, Picazo JJ, Pineda Solas V. Por qué hay más empiemas pediátricos en España? [Why are pediatric empyemas on the increase in Spain?]. An Pediatr (Barc). 2008 Feb;68(2):158-64. Spanish. doi: 10.1157/13116233. PMID: 18341884.
- Li ST, Tancredi DJ. Empyema hospitalizations increased in US children despite pneumococcal conjugate vaccine. Pediatrics. 2010 Jan;125(1):26-33. doi: 10.1542/peds.2009-0184. Epub 2009 Nov 30. Erratum in: Pediatrics. 2010 Feb;125(2):415. PMID: 19948570.
- Spencer DA, Cliff D. The changing epidemiology of parapneumonic empyema in children. Pediatrics and Child Health. 2008; 18(11):513‑518.
- Aziz A, Healey JM, Qureshi F, Kane TD, Kurland G, Green M, Hackam DJ. Comparative analysis of chest tube thoracostomy and video-assisted thoracoscopic surgery in empyema and parapneumonic effusion associated with pneumonia in children. Surg Infect (Larchmt). 2008 Jun;9(3):317-23. doi: 10.1089/sur.2007.025. PMID: 18570573.
- Guyon G, Allal H, Lalande M, Rodière M. Les pleurésies purulentes de l'enfant: expérience montpelliéraine [Pleural empyema in children: Montpellier's experience]. Arch Pediatr. 2005 Apr;12 Suppl 1:S54-7. French. doi: 10.1016/s0929-693x(05)80013-2. PMID: 15893240.
- Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995 Nov;108(5):1345-8. doi: 10.1378/chest.108.5.1345. PMID: 7587439.
- Brutsche MH, Tassi GF, Györik S, Gökcimen M, Renard C, Marchetti GP, Tschopp JM. Treatment of sonographically stratified multiloculated thoracic empyema by medical thoracoscopy. Chest. 2005 Nov;128(5):3303-9. doi: 10.1378/chest.128.5.3303. PMID: 16304276.
- Berlioz M, Haas H, Albertini M, Bastiani-Griffet F, Kurzenne JY. Intérêt de la thoracoscopie dans les pleurésies purulentes de l'enfant de moins de quatre ans [Value of thoracoscopy in purulent pleuresies in children younger than four years]. Arch Pediatr. 2001 Feb;8(2):166-71. French. doi: 10.1016/s0929-693x(00)00179-2. PMID: 11232457.
- Donnelly LF, Klosterman LA. CT appearance of parapneumonic effusions in children: findings are not specific for empyema. AJR Am J Roentgenol. 1997 Jul;169(1):179-82. doi: 10.2214/ajr.169.1.9207521. PMID: 9207521.
- Hilliard TN, Henderson AJ, Langton Hewer SC. Management of parapneumonic effusion and empyema. Arch Dis Child. 2003 Oct;88(10):915-7. doi: 10.1136/adc.88.10.915. PMID: 14500314; PMCID: PMC1719318.
- Kalfa N, Allal H, Lopez M, Saguintaah M, Guibal MP, Sabatier-Laval E, Forgues D, Counil F, Galifer RB. Thoracoscopy in pediatric pleural empyema: a prospective study of prognostic factors. J Pediatr Surg. 2006 Oct;41(10):1732-7. doi: 10.1016/j.jpedsurg.2006.05.066. PMID: 17011279.
- Padman R, King KA, Iqbal S, Wolfson PJ. Parapneumonic effusion and empyema in children: retrospective review of the duPont experience. Clin Pediatr (Phila). 2007 Jul;46(6):518-22. doi: 10.1177/0009922806299096. PMID: 17579104.