More Information
Submitted: January 05, 2021 | Approved: January 27, 2021 | Published: January 28, 2021
How to cite this article: Overbeck TR, Wenleder SHP, Danner BC, Körber W, Toepelt K, et al. Induction therapy with Erlotinib (E) and Gemcitabine/Platinum (GP) in stage III NSCLC. J Pulmonol Respir Res. 2021; 5: 001-018.
DOI: 10.29328/journal.jprr.1001018
Copyright License: © 2021 Overbeck TR, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Induction; Erlotinib; NSCLC; Stage III; Lung cancer
Induction therapy with Erlotinib (E) and Gemcitabine/Platinum (GP) in stage III NSCLC
Tobias R Overbeck1,2*, Stefan HP Wenleder1*, Bernhard C Danner2,3, Wolfgang Körber2,4, Karin Toepelt5, Bernhard Hemmerlein6,7, Christina Perske6, Markus Falk8, Markus Tiemann8, Claudia Tomala1, Elke Stitz1 and Frank Griesinger9
1Department of Hematology and Medical Oncology, University Hospital Göttingen, Göttingen, Germany
2Lung Tumor Center University of Göttingen and Göttingen Comprehensive Cancer Center (G-CCC), Germany
3Department of Thoracic and Cardiovascular Surgery, University Hospital Göttingen, Göttingen, Germany
4Abteilung Pneumologie, Beatmungsmedizin und Schlaflabor, Evangelisches Krankenhaus Weende, Göttingen, Germany
5MVZ University Clinic Köln, Köln, Germany
6Institute of Pathology, University Hospital Göttingen, Göttingen, Germany
7Institute of Pathology, Helios Klinikum Krefeld, Krefeld, Germany
8Institute of Hematopathology Hamburg, Hamburg, Germany
9Department of Hematology and Oncology, Department of Internal Medicine-Oncology, Pius-Hospital Medical Campus, University of Oldenburg, Oldenburg, Germany
*These authors contributed equally
*Address for Correspondence: Dr. Tobias R Overbeck, MD, Department of Hematology and Medical Oncology, University Hospital Göttingen, Robert-Koch-Str. 40 37075 Göttingen, Germany, Tel: +49 551 39-8943; Tel/Fax: +49 551-39-12534; Email: [email protected]
Background: In 2004 we started a phase II trial in non-small lung cancer (NSCLC), stage III, with erlotinib followed by a combination with a platinum-based doublet in unselected patients to identify molecular subgroups benefitting from an EGFR targeting approach.
Patients and methods: Induction with erlotinib (E, 150 mg, d1-42) was followed by three cycles of gemcitabine (G, 1250 mg/m², d1+d8, q3w) and cisplatin (P, 80 mg/m², d1, q3w). Patients with at least stable disease after E were treated with a GP + E combination. Induction was followed by surgery and radiation. The trial was conducted as a prospective, multi-center, open label, exploratory phase II study to determine pathological response rate (pRR), as well as secondary endpoints disease free survival (DFS) and overall survival (OS).
Results: Of 38 prescreened patients 16 were included in the main study. Due to slow recruitment the study had to be terminated early. Combination of E and GP was well tolerated, surgery was feasible after induction therapy in 12 of 16 patients, 7/12 (58%) patients had a major pathological response (MPR). Median overall survival for patients with MPR was 57.7 months (confidence interval (CI), 37.4 to 78.0; n = 7) and for patients without MPR 11.9 months (CI, 6.4 to 17.4; n = 5). 2/16 patients had an epidermal growth factor receptor (EGFR) mutation.
Conclusion: Before discovery of distinct molecular mechanisms in NSCLC our study was an attempt to identify clinical and pathological subgroups that would benefit from E induction. Two patients with an EGFR mutation were identified. MPR was a predictor of long term disease free and overall survival.
AUC: Area Under The Curve; BRAF: B Rat Fibrosarcoma; CI: Confidence Interval; CR: Complete Remission; cRR: Clinical Response Rate; CT: Computed Tomography; CTCAE: Common Toxicity Criteria for Adverse Events; DFS: Disease Free Survival; DNA: Deoxyribonucleic Acid; E: Erlotinib; ECOG PS: Eastern Co-Operative Oncology Group Performance Status; EFS: Event-Free Survival; EGFR: Epidermal Growth Factor Receptor; EML4-ALK: Echinoderm Microtubule-Associated Protein-Like 4 - Anaplastic Lymphoma Kinase; 18F-FDG-PET: 18F-Fluordesoxyglucose Positron Emission Tomography; 18F-FDG-PET-CT: 18F-Fluordesoxyglucose Positron Emission Tomography-Computed Tomography; FISH: Fluorescence In Situ Hybridization; G: Gemcitabine; GP: Gemcitabine/Platinum; Gy: Gray; HR: Hazard Ratio; ITT: Intent-To-Treat; Junker RG: Junker Regression Grade; KRAS: Kirsten Rat Sarcoma Viral Oncogene Homologue; MGB: Minor Groove Binder; MET: Mesenchymal-Epithelial Transition; MPR: Major Pathological Response; MRI: Magnetic Resonance Imaging; mRR: Metabolic Response Rate; NCI-CTC: National Cancer Institute Common Toxicity Criteria; NSCLC: Non-Small Cell Lung Cancer; ORR: Overall Response Rate; OS: Overall Survival; P: Platinum; pCR: Pathologic Complete Response; PCR: Polymerase Chain Reaction; PD: Progressive Disease; PD-L1: Programmed Death-Ligand 1; PFS: Progression Free Survival; PR: Partial Response; pRR: Pathological Response Rate; Q3w: Three-Weekly; RECIST 1.0: Response Evaluation Criteria In Solid Tumors, Version 1.0; SD: Stable Disease; SUV: Standardized Uptake Value; TKI: Tyrosine Kinase Inhibitor; TP53: Tumor Suppressor Gene P53; TPS: Tumor Proportion Score; UICC: Union For International Cancer Control
Therapeutic concepts in locally advanced non-small cell lung cancer (NSCLC) at stages IIIA/B remain a major interdisciplinary challenge. While the standard of care for localized lung cancer stages I and II is surgery, concomitant chemo-radiotherapy is the treatment of choice in locally advanced stages IIIA/B in most institutions. However, also tri-modality treatment, including chemotherapy, radiotherapy and surgery, has been studied in a number of clinical trials showing feasibility of this approach. Unfortunately, the majority of stage IIIA/B patients develops distant metastases or local relapse within several months of treatment. Median survival of 9-12 months only and 5-year survival rates of about 10% - 20% can be achieved by chemo-radiotherapy and multimodal treatment. Adjuvant chemotherapy has shown an absolute increase in 5-year survival of 4% with platinum-based doublet regimens irrespective of additional radiotherapy in stages IB-IIIA and is considered standard of care after radical surgical resection [1]. However, until 2017, no additional systemic treatment after chemo-radiotherapy had shown a survival benefit in stages IIIA and IIIB. It has been recently shown, that patients benefit from anti-programmed death-ligand 1 (anti-PD-L1) blockage with durvalumab after chemo-radiotherapy in stages IIIA and IIIB for the endpoints progression free survival (PFS) and overall survival (OS) (with a PD-L1 expression of > 1% tumor proportion score (TPS)). Durvalumab has therefore been approved for consolidation treatment throughout Europe [2].
Also, recently immunotherapy containing neo-adjuvant regiments were published with notable results in pathologic response after induction therapy [3-5].
At time of design of the study the effect of neo-adjuvant therapy prior to surgery had been evaluated in a series of phase II trials, as well as in prospective randomized trials. Two of the randomized trials with pre-operative chemotherapy showed an advantage in survival of the experimental arm [6-8], whereas a third trial did not show an advantage in stage IIIA patients [9].
Neo-adjuvant concepts can be classified in 3 categories: chemotherapy alone, concomitant chemo-/radiotherapy and sandwich approach with chemotherapy followed by chemo-/radiotherapy. Current data show a rate of surgical resectability of 40% - 80% and an advantage in survival compared to historical control data. The sandwich concept has been compared to chemotherapy alone in a randomized phase III trial. The results of this trial showed no differences in all endpoints, especially concerning the rate of surgical resectability, progression free survival and overall survival [10] 2014, the Meta-analysis Collaborative Group analyzed 15 randomized controlled trials and detected a significant benefit of preoperative chemotherapy on survival with a hazard ratio of 0,87 and a 13% reduction in the relative risk of death with an absolute survival improvement of 5% at 5 years. There was no clear evidence of a difference in the effect on survival regarding additional postoperative radiotherapy [11]. Horita, et al. reported in a pooled meta-analysis for a subgroup of stage III only patients comparing preoperative chemotherapy plus surgery and surgery alone a hazard ratio of 0.77 (95% confidence interval (CI), 0.68-0.87; p < 0.001) for overall survival favoring combination therapy. By comparison, for adjuvant systemic therapy the pooled hazard ratio was 0.83 in the Lung Adjuvant Cisplatin Evaluation (surgery plus postoperative chemotherapy vs. surgery alone) (12, 13). Felip et al. investigating early stages (IA > 2 cm till IIIA, T3N1, Union for International Cancer Control (UICC), 6th edition (2002), have not found any statistically significant differences in disease-free survival with the addition of preoperative or adjuvant chemotherapy to surgery. Only 1.5% (9/619) of all patients in this study were diagnosed at stage IIIA, so this study is not representative for locally advanced disease [14].
One of the best predictive parameters for long term survival is pathologic response after induction therapy. This has amongst others been shown by Betticher, et al. in patients with pathologically proven N2 disease at baseline and downstaging to N0-1 at surgery. Also this has been shown by the group of Thomas and Junker, suggesting that patients with less than 10% vital tumor cells and signs of therapy induced fibrosis have a more favorable survival than patient with no pathologic response[15]. This nodal downstaging significantly prolonged event-free survival (EFS) and overall survival (OS) [16]. Also some early data suggested that metabolic response after induction therapy assessed by 18F-fluordesoxyglucose positron emission tomography (-computed tomography) 18F-FDG-PET(-CT) might serve as a surrogate parameter for histologic regression of tumors and mediastinal lymph nodes after induction therapy [17].
At the time of the design of the study, gefitinib (G) and erlotinib (E) had been investigated in an all-comer population, showing a modest survival benefit. First publications of molecular mechanisms of “super responders” were available, but it was not clear whether additional patients might benefit to a large extent from epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) therapy. Also, the combination of tyrosine kinase inhibitor (TKI) and chemotherapy studied in the TALENT and TRIBUTE trials had not been published.
Therefore, the aim of the CHALLENGE trial was to further optimize chemotherapy by adding erlotinib (E) for patients benefiting from erlotinib single agent induction therapy. This approach was intended to identify additional biomarkers based on clinical response. The combination of three-weekly cycles of gemcitabine (G, 1250 mg/m², d1+d8) and cisplatin (P, 80 mg/m², d1) and erlotinib (E, 150 mg/day) has been administered to more than 500 patients in a large randomized phase III-trial [18]. Therefore, this approach was deemed feasible in this patient population.
Patient characteristics
Patients with potentially operable NSCLC stage IIIA/IIIB (UICC, 6th edition, (2002)) were enrolled in a prospective, multicenter, phase II trial of induction systemic therapy consisting of E followed by GE plus/minus E. All patients had passed a tumor board of a thoracic surgeon, pneumonologist, medical oncologist, radiologist, radio-oncologist, and pathologist confirming stage and eligibility for this study.
Patients were required to have mediastinoscopically or by bronchoscopic mediastinal samples confirmed, untreated, potentially operable stage IIIA or IIIB NSCLC.
Further inclusion criteria were age ≥ 18 years, Karnofsky performance status ≥ 80% or ECOG PS (Eastern Co-operative Oncology Group performance status) ≤ 1, adequate hematological laboratory parameters: hemoglobin ≥ 10 g/dl, white blood count ≥ 3000/μl, platelets ≥ 100000/μl, adequate hepatic laboratory parameters: Bilirubin ≤ 2.0 mg/dl, Aspartat-Aminotransferase ≤ 2x upper normal limit), adequate renal laboratory parameters, creatinine ≤ 1.5 mg/dl, creatinine clearance > 60 mg/ml/min, normal cardiac function defined by left ventricular ejection fraction > 49% (echocardiography), electrocardiogram without significant signs of cardiac arrhythmias, forced expiratory volume in 1 second ≥ 1.2 calculated, or determined by perfusion-ventilation scintigraphy and/or spiroergometry, diffusion capacity ≥ 70% (corrected by the alveolar volume) or 15 ml/min*kg as determined by spiroergometry, functional operability, agreement by the patient to use an effective method of contraception, negative pregnancy test for women of childbearing potential unless they are postmenopausal at baseline. Provision of informed consent according to local regulatory requirements including consent to the molecular-genetical analysis of tissue samples prior to any protocol specific treatment, pathologically confirmed diagnosis of NSCLC, stage IIIA (T1-3; N2) or IIIB (T1-3; N3), T4 (as defined by solitary metastasis in the same lobe as primary tumor; N2/N3) (UICC, 6th edition, 2002) and measurable lesions according to Response Evaluation Criteria In Solid Tumors, version 1.0 (RECIST 1.0). The following histological tumor types were eligible: squamous cell carcinoma, adenocarcinoma, including adenocarcinoma with bronchoalveolar differentiation, large cell carcinoma, including large cell carcinoma with neuroendocrine differentiation, mixed cell carcinoma without small cell fraction.
Exclusion criteria were pregnancy or lactation period, presence of a pancoast tumor and/or a T4 tumor other than defined in the inclusion criteria and/or a bronchoalveolar carcinoma and/or a mixed cell carcinoma including small cell fractions and/or distant metastases, involvement of supraclavicular lymph nodes, other co-existing malignancies or malignancies diagnosed within the last 5 years, with the exception of a carcinoma in situ of the cervix or non-melanomatous skin cancer. Previous radio- and/or chemotherapy within the last five years, resection of primary malignancy, treatment with an investigational new drug, currently or within 28 days prior to enrollment, and/or participation in another clinical trial, currently or during the last 12 weeks, and/or previous participation in this study, history of a mental disorder or condition such as to interfere with the patient’s ability to understand the requirements of the study, patients with any clinically significant disease that in the opinion of the investigator is likely to put the patient at risk or to interfere with the evaluation of the patient’s safety and of the study outcome. This included, but was not limited to any known significant ophthalmologic abnormalities of the surface of the eye, immediate need for therapeutic intervention, clinically significant cardiac disease or myocardial infarction within the last 6 months, uncontrolled hypertension, interstitial pneumonia or extensive or symptomatic interstitial fibrosis of the lung; pleural effusion or ascites, which cause respiratory compromise, any other active or uncontrolled infection, organ allografts, a history or presence of any central nervous system disorder or psychiatric disability judged by the investigator to be clinically significant and/or interfering with compliance of oral drug intake inability to swallow pills, concomitant coumadin/phenprocoumon use, alcohol and/or drug abuse, patients who cannot be regularly observed for psychological, sociological or geographical reasons or other concomitant conditions not permitting adequate follow-up and compliance to the protocol.
The authors assure that all clinical investigations are conducted in accordance with the Declaration of Helsinki. Approval of the ethics committee (Ethikkommission der Universitätsmedizin Göttingen, No. 30/3/04) was given.
Trial design and treatment plan
The trial was conducted as a prospective, multi-center, open label, exploratory phase II study to determine the pathological Response Rate (pRR), evaluated with morphometry, of a gemcitabine/cisplatin (GP) induction chemotherapy with or without erlotinib (E) treatment, where E and GP treatment was administered to patients without progressive disease during an initial E induction treatment.
Secondary objectives were to determine the clinical Response Rate (cRR) evaluated by computed tomography (CT) and to determine the metabolic Response Rate (mRR) by 18F-FDG-PET of 6 week erlotinib induction treatment followed by a gemcitabine/cisplatin chemotherapy with or without erlotinib. Metabolic remission was assessed by the sum of the products of the largest PET-lesion diameter and the maximum standardized uptake value (SUV) of all lesions (primary tumor as well as lymph nodes) and metabolic response was defined as a reduction of > 85%. Further objectives were to determine the rate of surgical resection, event-free survival (EFS, where events were defined as progression on chemo induction, recurrent disease or death) and overall survival (OS), as well as to determine the toxicity of the regimen and to correlate the results of the microarray analyses with the pathological and clinical outcome of the study.
Tyrosine kinase inhibitor, erlotinib
Erlotinib inhibits selectively and reversibly the human EGFR tyrosine kinase and EGF-dependent proliferation of cells and blocks cell-cycle progression in the G1 phase. The dose of erlotinib (E) with 150 mg/day was based on pharmacokinetic parameters as well as the safety and tolerability profile at this dose level in phase I trials in advanced, heavily pretreated cancer patients (19).
Patients were planned to receive erlotinib during the initial tyrosine kinase inhibitor (TKI) only induction therapy phase at a dose of 150 mg erlotinib once daily for six weeks. During the induction chemotherapy phase all patients with at least stable disease (SD) after the initial erlotinib induction were planned to continue treatment with a dose of 150 mg erlotinib once daily, except on days when chemotherapy was administered. Dose interruption was allowed for a maximum of 2 weeks in the event of any toxicity. Perioperative and during radiotherapy no erlotinib was given.
Consolidation therapy with erlotinib started on day 84 after the beginning of the 6 weeks adjuvant radiotherapy (d42 after the end of radiotherapy) with a dose of 150 mg erlotinib once daily up to 12 months in all patients with at SD at the first restaging (days 43/44).
Dose reductions were allowed. Once a patient has had a dose reduction for toxicity, the dose was not allowed to be re-escalated (except for patients with rash grade 3 that improves to rash ≤ Grade 2). Erlotinib dosage modification followed criteria for erlotinib related toxicities as well as guidelines for their management.
Chemotherapy, platin and gemicitabine
Cisplatin interferes with DNA replication and is backbone of systemic approaches in lung cancer therapies. Gemcitabine is a pyrimidine analogue and induces an inhibition of DNA synthesis leading to cell death. Phase I studies for the combination of erlotinib with gemcitabine/cisplatin have been published. The combination was applied safely at three-weekly courses of gemcitabine and cisplatin and erlotinib daily [18,20].
A standard chemotherapy protocol consisting of 3 cycles of gemcitabine and cisplatin was used: gemcitabine 1250mg/m² was administered on days 1 and 8 of each chemotherapy cycle three-weekly (q3w) as 30 min i.v. infusion. Cisplatin 50mg/m² was administered on days 1 and 8 of each chemotherapy cycle (q3w) as 1h i.v. infusion. Doses were modified for hematologic and non-hematologic toxicities. In case of toxicities dose adjustment followed recommended guidelines. Toxicity grading was according to National Cancer Institute Common Toxicity Criteria (NCI-CTC), version 2.0. In case of clinical contraindication to cisplatin, e.g. creatinine clearance below 60 ml/min or a loss of hearing - more than 50% decrease compared to baseline, cisplatin treatment was planned to be discontinued and carboplatin (area under the curve (AUC) 5, day 1, every 3 weeks for 3 cycles) was administered instead.
Surgery
In all patients, having stage IIIA/B disease without progressive disease after induction chemotherapy and functional operability, resection of the primary tumor and radical lymphadenectomy was performed. During surgery tumor material was collected for pathological evaluation. Operability was assessed in an interdisciplinary tumor board prior to surgery, involving specialists in thoracic surgery, pneumonology, medical oncology, radiotherapy and radiology. Surgery was performed by a surgeon experienced in surgical strategies following induction chemotherapy.
During surgery, lobectomy, bilobectomy or pneumonectomy, rapid section analysis of the more proximal bronchus resection was performed to guarantee R0 resection. After anatomical resection of the tumor bearing lobe/lung, a complete ipsilateral lymph node resection from caudally to cranially, starting at the pulmonary ligament followed by the paraesophageal, bifurcation, tracheobronchial, paratracheal lymph nodes up the lymph nodes of the anterior mediastinum was performed. At the left side, the subaortal lymph nodes have been resected.
Postoperative therapy, radiotherapy
Postoperative radiotherapy was administered to all resected patients involving primary tumor and mediastinum, in case of a N3 disease, including the supraclavicular region. A daily dose of 2 Gray (Gy) was administered 5 days a week, in patients with R0 resection to a cumulative dose of 50 Gy, an in R1/R2 resection of 60 Gy. Prophylactic cranial irradiation was optional and considered in patients with R0 resection and metabolic or histopathologic response and planned concomitant with mediastinal irradiation at a dose of 2 Gy/fraction and 15 fractions.
Evaluations
During erlotinib induction phase (days 1 - 42) physical examination, ECOG performance status and laboratory assessment including full differential blood count and blood chemistry were performed every two weeks. During chemotherapy induction phase (days 45 - 107) these tests were performed on a weekly base as well as before surgery and radiotherapy. During consolidation with erlotinib these tests were performed at least every 3 months.
Response to induction therapy was evaluated by RECIST 1.0 and pathological response was defined by regression scale according to Junker as follows [15].
Grade I: no tumor regression or only spontaneous tumor regression in the sections of the primary lesion and mediastinal lymph nodes; Grade II: morphologic evidence of therapy-induced tumor regression with > 10% residual tumor cells in the sections of the primary lesion and/or mediastinal lymph nodes presenting more than focal microscopic disease (grade IIa) or < 10% residual tumor cells in the sections of the primary lesion and/or mediastinal lymph nodes presenting focal microscopic disease (grade IIb); and Grade III: complete tumor regression with no evidence of vital tumor tissue in the sections of the primary lesion and mediastinal lymph nodes.
Molecular pathology
From formaldehyde-fixed paraffin-embedded tissue blocks microtome sections were prepared (5 µM) and stained with hematoxylin-eosin for pathological evaluation of tumor content. Tumor cells were microdissected either manually or by laser-guided microdissection (Leica LMD6500).
Deoxyribonucleic acid (DNA) was extracted using NucleoSpin® Tissue Kit (Macherey-Nagel).
Mutations in EGFR (exons 18-21), Kirsten rat sarcoma viral oncogene homologue (KRAS, codons 12/13/61 in exons 2/3) and tumor suppressor gene p53 (TP53, exons 5-9) were analyzed by direct Sanger sequencing. B rat fibrosarcoma (BRAF) mutations in codons V600 and G469 were analyzed using a laboratory developed real time polymerase chain reaction (PCR) with allele specific minor groove binder (MGB) probes. Echinoderm microtubule-associated protein-like 4 - anaplastic lymphoma kinase (EML4-ALK) translocations were tested by a lab developed PCR assay for detection of the most prevalent EML4 fusion sites of exons 2, 5, 13, 14, 17, 20 with ALK exon 20. Mesenchymal-epithelial transition (MET) amplification status was assessed immunohistochemically (antibody clone SP44, Zytomed) utilizing the automated Bond Max Platform (Menarini). Primers and Probes are listed in table 1.
| Table 1: Primers, Probes and Sequences. | |
| Primers/MGB-probes | Sequence (5´-3´) |
| Primers EGFR (exons 18-21) | |
| Exon 18 FOR | AGGGCTGAGGTGACCCTTG |
| Exon 18 REV | CCTGTGCCAGGGACCTTAC |
| Exon 19 FOR | TGTCATAGGGACTCTGGATCC |
| Exon 19 REV | GGGCCTGAGGTTCAGAGCC |
| Exon 20 FOR | CGAAGCCACACTGACGTGC |
| Exon 20 REV | CCCGTATCTCCCTTCCCTG |
| Exon 21 FOR | TTCTCTGTTTCAGGGCATGAAC |
| Exon 21 REV | GTGGGAAGGCAGCCTGGTC |
| Sequencing primers EGFR (only if distinct from PCR primers) |
|
| Exon 20 FOR | CGAAGCCACACTGACG |
| Exon 20 REV | GATTACCTTTGCGATCTG |
| Exon 21 FOR | TTCTCTGTTTCAGGGCAT |
| Exon 21 REV | GTGGGAAGGCAGCCT |
| Primers EML4-ALK | |
| EML4-Exon2 FOR | CTGAAGATCATGTGGCCTCAG |
| EML4-Exon5 FOR | ATGATAGCCGTAATAAATTGTCG |
| EML4-Exon13 FOR | TGGAGTCATGCTTATATGGAGC |
| EML4-Exon14 FOR | TGTGTTCACACTTTGTCAGATG |
| EML4-Exon17 FOR | ACTGTGCAGATTTTCATCCAAG |
| EML4-Exon20 FOR | ATCACACACCTTGACTGGTCC |
| ALK1 REV | CTTGCTCAGCTTGTACTCAG |
| Primer KRAS (Codon12/13) | |
| FOR | TATAAGGCCTGCTGAAAATGAC |
| REV | TTGTTGGATCATATTCGTCCAC |
| Primer KRAS (Codon61) | |
| FOR | CTCCCTTCTCAGGATTCCTAC |
| REV | TGGCAAATACACAAAGAAAGCC |
| Primers BRAF V600E | |
| FOR | TCTTCATGAAGACCTCACAGTA |
| REV | GCCTCAATTCTTACCATCCAC |
| Probes BRAF V600E | |
| WT | VIC-CTACAGTGAAATCT |
| Mut | 6FAM-CTACAGAGAAATCT |
| Primers BRAF G469A | |
| FOR | ACTTGGTAGACGGGACTCG |
| REV | TTACCATGCCACTTTCCCTTG |
| Probes BRAF G469A | |
| WT | 6FAM-CTGGATCATTTGGAACAGT |
| Mut | VIC-CTGGATCATTTGCAACAGT |
| Primers TP53 (Exons 5-9) | |
| Exon 5 FOR | GTGCCCTGACTTTCAACTCTG |
| Exon 5 REV | CAACCAGCCCTGTCGTCTC |
| Exon 6 FOR | CTCAGATAGCGATGGTGAGC |
| Exon 6 REV | ACCCCAGTTGCAAACCAGAC |
| Exon 7 FOR | CCCTGCTTGCCACAGGTCTC |
| Exon 7 REV | ACAGCAGGCCAGTGTGCAG |
| Exon 8 FOR | ACCTGATTTCCTTACTGCCTC |
| Exon 8 REV | GTGAATCTGAGGCATAACTGC |
| Exon 9 FOR | GCAGTTATGCCTCAGATTCAC |
| Exon 9 REV | AAGAGGTCCCAAGACTTAGTAC |
| Sequencing primers TP53 (only if distinct from PCR primers) |
|
| Exon 5 FOR | GCCCTGACTTTCAACTCTG |
| Exon 6 FOR | CAGATAGCGATGGTGAGC |
| Exon 6 REV | CCCAGTTGCAAACCAGAC |
| Exon 7 FOR | CTGCTTGCCACAGGTCTC |
| Exon 7 REV | AGCAGGCCAGTGTGCAG |
| Exon 8 FOR | CTGATTTCCTTACTGCCTC |
| Exon 8 REV | GAATCTGAGGCATAACTGC |
| Exon 9 FOR | CAGTTATGCCTCAGATTCAC |
| Exon 9 REV | GAGGTCCCAAGACTTAGTAC |
Statistical analysis
Data were described as numbers and percentages for categorical variables, and as median, mean +- standard deviation for continuous variables. Survival times were analyzed with the Kaplan-Meier method and for comparing the survival times across different groups, log rank test was applied. Overall survival (OS) was calculated on the date of first diagnosis to the date of last follow up or death. Event-free survival (EFS) was determined from the date of first diagnosis to the first occurring event: progression on chemo induction, recurrent disease or death. Patients who were still alive or were lost to follow-up were censored at the time of the last contact. Results were considered significant if p-values were less than 0.05. SPSS software (IBM Corp., IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY USA) was used for statistical analysis. Due to early termination of the study, results only can be descriptive.
Patients
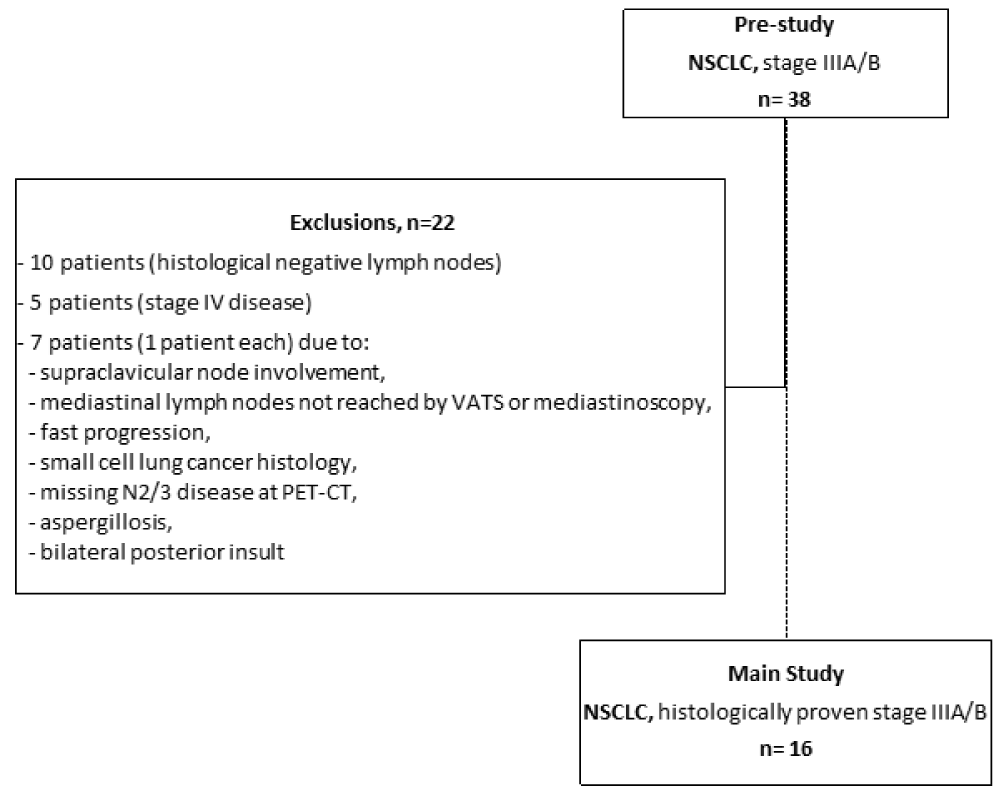
Between July 2004 and February 2007 we included 38 Caucasian patients with assumed NSCLC stage IIIA or IIIB disease by CT scan in a pre-study phase (screening) of this study at 5 German centers. Due to the following circumstances 22 patients were not included in the main study due to in- and exclusion criteria: histologically negative mediastinal lymph nodes (10 patients), stage IV disease (5 patients), and 1 patient each for supraclavicular nodal involvement, mediastinal lymph nodes not reached by video-assisted thoracoscopic surgery (VATS) or mediastinoscopy, fast progression, small cell lung cancer histology, missing N2/3 disease at 18F-FDG-PET-CT, aspergillosis, and bilateral posterior insult. Figure 1 shows numbers of patients in pre-study and main study phase and reasons of exclusion for the main study. Unfortunately, since one further participating center did not report sufficient data of included patients, data of these patients are not reported in this summary of our investigation.
Figure 1: Consort diagram. Depicted are numbers of patients in pre-study and main study phase and reasons of exclusion for the main study.
Eligible for the main study with histologically proven stage IIIA/B disease (UICC, 6th edition, 2002) remained 16 patients. Baseline characteristics of these patients (main study) are shown in table 2.
| Table 2: Patient Characteristics. | ||
| Patient Characteristics | N | % |
| Patients | 16 | 100 |
| Age (years) | ||
| Mean | 62.9 | |
| Median | 65 | |
| Range | 46 - 75 | |
| Under 65 years | 8 | 50 |
| Over 65 years | 8 | 50 |
| Gender | ||
| Female | 4 | 25 |
| Male | 12 | 75 |
| Histological Subtype | ||
| Adenocarcinoma | 7 | 43.75 |
| Squamous cell carcinoma | 8 | 50 |
| Mixed Adeno/Squamous | 1 | 6.25 |
| Grading | ||
| 1 | 1 | 6.25 |
| 2 | 10 | 62.5 |
| 3 | 2 | 12.5 |
| Unknown | 3 | 18.75 |
| T-status | ||
| T1 | 0 | 0 |
| T2 | 12 | 75 |
| T3 | 2 | 12.5 |
| T4 | 2 | 12.5 |
| N-status | ||
| N0 | 0 | 0 |
| N1 | 0 | 0 |
| N2 | 6 | 37.5 |
| N3 | 8 | 50 |
| Nx | 2 | 12.5 |
| Stage | ||
| IIIA | 5 | 31.25 |
| IIIB | 11 | 68.75 |
| Smoking Status | ||
| Current smoker | 9 | 56.25 |
| Former smoker | 4 | 25 |
| Never smoker | 2 | 12.5 |
| Unknown | 1 | 6.25 |
| ECOG Performance Status | ||
| 0 | 12 | 75 |
| 1 | 4 | 25 |
75% of all patients had a performance status ECOG PS 0 and 25% of 1. The median age was 65 years (range 46 to 75 years). Squamous cell carcinoma was the predominant subtype (50%) followed by adenocarcinoma (43.75%), mixed subtype was described in 6.25%. All patients had a mediastinoscopically confirmed N2 or N3 disease (14 patients) or T4 with mediastinal involvement (2 patients). Uni- or multilevel nodal mediastinal disease was summarized as N2 disease and not further categorized.
Systemic therapy
In total, 16 patients received 89.14% of planned erlotinib single therapy during induction therapy (3500 to 6300 mg, mean 5615.63 mg, median 6300 mg). 10 patients obtained 2 complete courses of erlotinib 150 mg orally (42 days) as monotherapy, cumulative dose was 6300 mg per patient, nine patients with stable disease (SD) or partial remission (PR), one patient with progressive disease (PD) at 1st restaging. 4 patients stopped erlotinib early due to progression on day 27, 30, 28, and 41, respectively, mean dose was 4725 mg (4050 mg to 6150 mg). 2 patients received erlotinib with a reduced dose due to diarrhea and rash in both patients and additionally fatigue in one of them, cumulative doses were 3500 mg (14 days 150 mg, paused for 7 days, 14 days 50 mg, 7 days 100 mg) and 4450 mg (14 days 150 mg, 5 days paused, 7 days 50 mg, 8 days 100 mg, 8 days 150 mg), respectively, both patients showed SD at 1st restaging.
All 16 patients were treated with a platinum based induction chemotherapy combined with gemcitabine. Mean doses for all 3 cycles were 91% for platinum as well as for gemcitabine. 3 Patients were treated upfront with carboplatin (AUC 5, day 1, every 3 weeks for 3 cycles) instead of cisplatin due to elevated serum creatinine in 2 cases, and persistent diarrhea and rash post erlotinib, respectively. One patient was switched to carboplatin AUC 5 at the 3rd cycle because of myocardial infarction, one other patient received carboplatin at the 2nd cycle with a 25% dose reduction due to hematotoxicity.
The median dose per administered cycle was 100 mg/m2 for cisplatin (range, 0 mg to 100 mg/m2, target dose 100 mg/m2), and AUC 5 (range AUC 3,75 to 5) for carboplatin, respectively. For gemcitabine the median dose was 2500 mg/m2 (range, 0 mg to 2500 mg/m2, target dose 2500 mg/m2).
Reasons for dose reductions were hematotoxicity and diarrhea. One patient showed fatigue, thrombocytopenia and renal impairment on day 8 of the 1st cycle, pneumonia on day 12, and a myocardial infarction on day 25. In this patient induction chemotherapy was stopped following day 1 of the first cycle. In one patient doses of cisplatin and gemcitabine were increased from cycle 1 to 3 from 50% to 75% to 100% per cycle, initial dose reduction of chemotherapy was due to diarrhea after erlotinib induction.
10 of 16 patients were not progressing (PR or SD) after erlotinib induction and were planned for platinum based gemcitabine induction chemotherapy combined with erlotinib 150 mg per day (except for days with chemotherapy). 8/10 patients received erlotinib and gemcitabine/platin chemotherapy, 7 with 100% of the planned erlotinib dose, 1 patient with a reduced dose of 100 mg due to diarrhea and rash during single agent erlotinib induction. 2 patients having experienced diarrhea and rash during erlotinib induction did not start erlotinib in combination with chemotherapy.
Erlotinib maintenance: Of 10 patients having achieved at least stable disease (PR and SD) at 1st restaging after erlotinib induction, 7 patients completed chemotherapy induction, surgery and postoperative radiotherapy.
4 of 7 patients started maintenance therapy with erlotinib. Both patients with known EGFR mutations received erlotinib, patient 06_014 (common mutation, exon 19, p.E746_R748del) 150 mg per day for 347 days and patient 01_034 (uncommon mutation, exon18, p.P694L) 150 mg per day for 31 days and 100 mg per day for 333 days. Patient 01_003 (EGFR wildtype) got erlotinib 150 mg for 192 days and stopped with progressive disease. The fourth patient (01_007, EGFR wildtype) got 150 mg erlotinib for 89 days and 100 mg for 70 days, interruption and dose reduction followed diarrhea and rash.
Response
Erlotinib induction: Overall response rate (ORR) at 6 weeks per protocol or at an earlier time point for those that had progressive disease or toxicity for all 16 patients was 12.5% (2/16 patients), including 2 partial (PR) and no complete remissions (CR). 8/16 patients (50.0%) had a stable disease (SD) and 6/16 patients (37.5%) showed progression (PD). Metabolic response rate (mRR) by PET scan was 25% (4/16 patients).
Cisplatin/gemcitabine induction: All 16 patients received platinum based combination therapy. Overall response rate (ORR) for all patients was 56.25% with 9/16 patients with PR and no patient with complete remission (CR). 4/16 Patients (25%) had SD and 2/16 patients (12.5%) PD with new metastases.
Patients with PD after Erlotinib induction (n = 6) responded with PR in 4 cases, with SD in one case and with further progression in another case. The second patient with progression after chemotherapy had SD after erlotinib induction.
One patient died on day 31 of the 1st cycle of chemo induction after having completed only day 1 of the 1st cycle followed by fatigue, thrombocytopenia and renal impairment on day 8, pneumonia on day 12, and a myocardial infarction on day 25. This patient’s best response to erlotinib induction was SD. For calculation of clinical response rate (cRR) and metabolic response rate (mRR) this patient was included as part of the intent-to-treat group (ITT).
cRR by CT scan after chemotherapy plus/minus erlotinib induction was 56,3% (9/16 patients), mRR by PET scan was 62,5% (10/16 patients). No complete remission was seen at any time.
Surgery: 13/16 patients with at least stable disease after chemotherapy at 2nd CT scan were intended for surgery. 12 of these 13 patients underwent thoracotomy and resection. One of these 13 patients was not estimated to be adequate for R0 resection, best response after erlotinib induction and chemotherapy was SD, respectively. Therefore, this patient was treated with concomitant chemo-radiotherapy following induction therapy. Two of 16 patients showed PD with newly diagnosed metastatic disease at restaging and were not eligible for surgery, 1 of 16 patients died due to myocardial infarction during the chemotherapy phase. Surgical resection rate of the ITT population therefore was 75% (12/16).
7/12 patients underwent pneumonectomy, 2 left sided (16.7%) and 5 right sided (41.7%). 5/12 patients underwent lobectomy, 4 left sided (33.3%) and 1 patient right sided (8.3%).
R0 resection was achieved in 10 patients (1/10 with carcinomatous lymphangiosis in the proximal part of the resected bronchus), 2/12 patients had R1 resection.
Pathologic response: In 12 patients, resection material was collected. 9/12 patients had a preoperative PR: 5 of these 9 patients showed a Junker regression grade (RG) IIb, 4 of these 9 patients had a Junker RG IIa. 2/12 patients had a preoperative SD and showed Junker RG IIb. 1/12 patients had PD after Erlotinib and SD after chemotherapy and Junker RG I.
In 15/16 patients molecular alterations were successfully studied. 2/15 patients had EGFR mutations, one patient with a common activating EGFR mutation (patient 06-014, exon 19 deletion, p.E746_R748del), and another patient with an uncommon exon 18 mutation (patient 01-034, exon 18, p.P694L). Further tests included fluorescence in situ hybridization (FISH) testing for EML4-ALK, sequencing for TP53, BRAF and KRAS mutations, and immunohistochemistry for MET. Table 3 shows results for molecular testing and best response following erlotinib and chemotherapy +- erlotinib induction, resection status, Junker regression grade and outcome (overall and event-free survival). One of only two partial remissions following erlotinib induction was observed in a patient with an exon 19 deletion. The other partial remission was noted in a patient with wildtype status in exon 19 and 21, but insufficient tumor material for further analysis including exons 18 and 20 as well as for KRAS and BRAF alterations, ALK or TP53. So in this patient no molecular target could be identified elucidating response to TKI monotherapy. However, it cannot be ruled out that this patient had an activating EGFR mutation that led to PR after erlotinib induction.
| Table 3: Molecular testing and outcome. | ||||||||
| Patient-ID | EGFR mutation (Exon 18-21) |
Non-EGFR alterations (tested: EML4-ALK, KRAS (Codon12, 13, 61), TP53, BRAF, cMET-IHC) |
Best response, E induction | Best response, GP + / - E | Resection status, R |
Junker regression grade, RG | Event-free survival, EFS (months) | Overall survival, OS (months) |
| 01-001 | WT | KRAS (Codon12, G34T), TP53 (Exon5, p.G154V), cMET 3+ | PD | PR | R0 | IIb | 168,4+ | 168,4+ |
| 01-003 | WT | TP53 (Exon8, p.G279E), cMET 3+ | SD | PR | R0 | IIb | 14,3 | 15,9 |
| 01-005 | WT (exons 19 and 21), ukn (exons 18 and 20) |
ukn for ALK, KRAS, TP53, BRAF; cMET 1+ | PR | PR | R0 | IIb | 8,0 | 8,0 |
| 01-007 | WT | KRAS (Codon12, G35T), cMET 3+ | SD | SD | R1 | IIb | 163,5+ | 163,5+ |
| 01-008 | WT | TP53 (Exon8, pR306fs*), cMET 2+ | SD | SD | R0 | IIb | 10,7 | 57,7 |
| 01-009 | WT | WT; cMET 0+ | PD | PR | R0 | IIa | 11,9 | 11,9 |
| 01-011 | WT | TP53 (Exon5, p.R181P & Exon6, p.R196Q), cMET 2+ | PD | PR | R0 | IIa | 8,0 | 9,4 |
| 06-014 | Exon19 (p.E746_R748del) | WT; cMET 3+ | PR | PR | R0 | IIb | 50,6 | 72,8 |
| 11-018 | WT | TP53 (Exon7, p.C229Y), cMET 0+ | PD | SD | R0 | I | 11,3 | 14,3 |
| 01-026 | ukn | ukn | SD | PD | (no surgery due to PD) |
- | 4,3 | 14,2 |
| 01-031* | WT | TP53 (Exon5, p.E180 & Exon8, p.E273H), cMET 0+ | PD | PD | (no surgery due to PD) |
- | 1,8 | 11,1 |
| 01-034 | Exon18 (p.P694L) | (TP53 n.a.), cMET 0+ | SD | PR | R0 | IIb | 24,9 | 49,8 |
| 10-042 | WT | TP53 (homozygous Exon5, p.G154fs14*), cMET 0+ | PD | PR | R1 | IIa | 7,9 | 7,9 |
| 09-046 | ukn | ukn | SD | (death before reevaluation) | (no surgery due to PD) |
- | 3,3 | 3,3 |
| 09-048 | WT | (TP53 n.a.), cMET 1+ | SD | PR | R0 | IIa | 10,4 | 13,2 |
| 06-049 | ukn | ukn | SD | SD | (no surgery due to doctor’s decision) |
- | 4,1 (censored) | 20,8 |
| Patient-ID: Patient-Identification; EGFR: Epidermal Growth Factor Receptor; EML4-ALK: Echinoderm Microtubule-Associated Protein-Like 4 - Anaplastic Lymphoma Kinase; KRAS: Kirsten Rat Sarcoma Viral Oncogene Homologue; TP53: Tumor Suppressor Gene P53; BRAF: B Rat Fibrosarcoma; cMET-IHC: Tyrosine-Protein Kinase Mesenchymal-Epithelial Transition – Immunohistochemistry; E: Erlotinib; GP: Gemcitabine/Platinum; R: Resection Status (R0: No Cancer Cells Seen Microscopically At The Primary Tumor Site; R1: Cancer Cells Present Microscopically at the Primary Tumor Site); RG: Regression Grade; EFS: Event-Free Survival; OS: Overall Survival; WT: Wild Type; PD: Progressive Disease; PR: Partial Response; SD: Stable Disease; Ukn: Unknown; *Patient 01-031 Showed At 1st Restaging Progression To Stage IV, Confirmed At 2nd Restaging. | ||||||||
Radiotherapy: Postoperative radiotherapy was administered to all 12 patients following resection. Patients with R0 resection were administered radiotherapy for 5 days per week to a cumulative dose of at least 50 Gy. 7/12 patients (58.3%) received fractions of 2 Gy per day up to 50 Gy. Two other patients (2/12; 16.7%) got a cumulative dose of 54 Gy in 1.8 Gy fractions. Two patients received 60 Gy (2.0 Gy fractions), one following R1 resection, another patient with R0 status but carcinomatous lymphangiosis in the resected bronchus. One further patient (8.3%) with R1 resection obtained a cumulative dose of only 52 Gy instead of 60 Gy for no known reasons. 2 Patients (16.7%) with R0 resection got additional prophylactic irradiation of the whole brain with 30 Gy.
Toxicity: All 16 patients were assessable for toxicity, results are depicted in table 4. Adverse events were depicted corresponding their occurrence during therapy. Grade 3 or 4 hematologic toxicity was seen only whilst the period of platinum based combination chemotherapy, i.e. anemia in 18,8% of all patients, leukocytopenia (25%) and thrombocytopenia (31,3%). Grade 3 or 4 non-hematologic toxicity were acute myocardial infarction (2 patients) and pneumonia (5 patients). Each one patient showed grade 3 or 4 toxicity for radiation pneumonitis, tachycardia, deep venous thrombosis, peripheral arterial occlusive disease, diarrhea and vomiting.
| Table 4: Non-Hematologic and hematologic toxicity. Numbers of patients at risk. E induction, n = 16; GP + /- E, n = 16; surgery, n = 12; radiotherapy, n = 12; E maintenance, n = 4. | ||||||||||||
| Phase of therapy | E induction | GP + / - E | Surgery | Radiotherapy | E maintenance | all | ||||||
| Toxicity grade (CTCAE 2.0) | 1-2 | 3-4 | 1-2 | 3-4 | 1-2 | 3-4 | 1-2 | 3-4 | 1-2 | 3-4 | 1-2 | 3-4 |
| Non-hematologic toxicity | ||||||||||||
| Acute myocardial infarction | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Allergic exanthema | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Alopecia | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Arthralgia | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Bleeding | 4 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 10 | 0 |
| Bronchitis | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cephalgia | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cholecystitis | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Conjunctivitis | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Constipation | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Cough | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 |
| Cutanous infection | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 |
| Cystitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 |
| Deep veinous thrombosis | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 |
| Depression | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 | 0 |
| Diarrhea | 9 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 1 |
| Dizzyness | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Dry mouth | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Dry skin | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Dysphagia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Dyspnea | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 6 | 0 |
| Edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Esophagitis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Exanthema | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 |
| Fatigue | 4 | 0 | 6 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 11 | 0 |
| Fever | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 6 | 0 |
| Gastritis | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 4 | 0 |
| Headache | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 0 |
| Herpes labialis | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Hot flushes | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Hypaesthesia | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Inappetence | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 |
| Increased creatinin level | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Infection | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| Insomnia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Itching | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Nausea | 2 | 0 | 9 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 13 | 0 |
| Night sweat | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Nykturia | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Pain | 2 | 0 | 4 | 0 | 3 | 0 | 2 | 0 | 1 | 0 | 12 | 0 |
| Paresthesia | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 3 | 0 |
| Peripheral arterial occlusive disease | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Pneumonia | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 5 |
| Radiation pneumonitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Rash | 8 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 11 | 0 |
| Systremma | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Tachycardia | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Tinnitus | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Vertigo | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Vomiting | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 |
| Weakness | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Weight loss | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 |
| Hematologic toxicity | ||||||||||||
| Anemia | 12 | 0 | 13 | 3 | 10 | 0 | 9 | 0 | 2 | 0 | 46 | 3 |
| Leukocytopenia | 0 | 0 | 8 | 4 | 1 | 0 | 0 | 0 | 1 | 0 | 10 | 4 |
| Thrombocytopenia | 0 | 0 | 8 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 9 | 5 |
| Sum, Non-hematologic toxicity | 51 | 3 | 48 | 3 | 19 | 3 | 15 | 4 | 15 | 0 | 148 | 13 |
| Sum, Hematologic toxicity | 12 | 0 | 29 | 12 | 12 | 0 | 9 | 0 | 3 | 0 | 65 | 12 |
Overall and event-free survival: By July 2018, 14 of 16 patients (87.5%) had died with a median survival of 14.2 months (range 3.3 to 168.4 months), 5 year and 10 year survival was 18.8%, and 12.5%, respectively. Two patients are long term survivors.
By July 2018, 13 of 16 patients (81.3%) have shown an event (2/16 with progression on E + GP + / - E induction, 1/16 with death before 2nd restaging, 7/16 with relapse of lung cancer and 3/16 with death without recurrent disease). One patient has terminated study treatment after having completed erlotinib and TKI-chemo-combination therapy resulting in SD without surgery due to doctor’s decision. 2/16 patients are still alive without recurrent disease. Median event-free survival was 10.7 months (range 1.8 to 168.4 months).
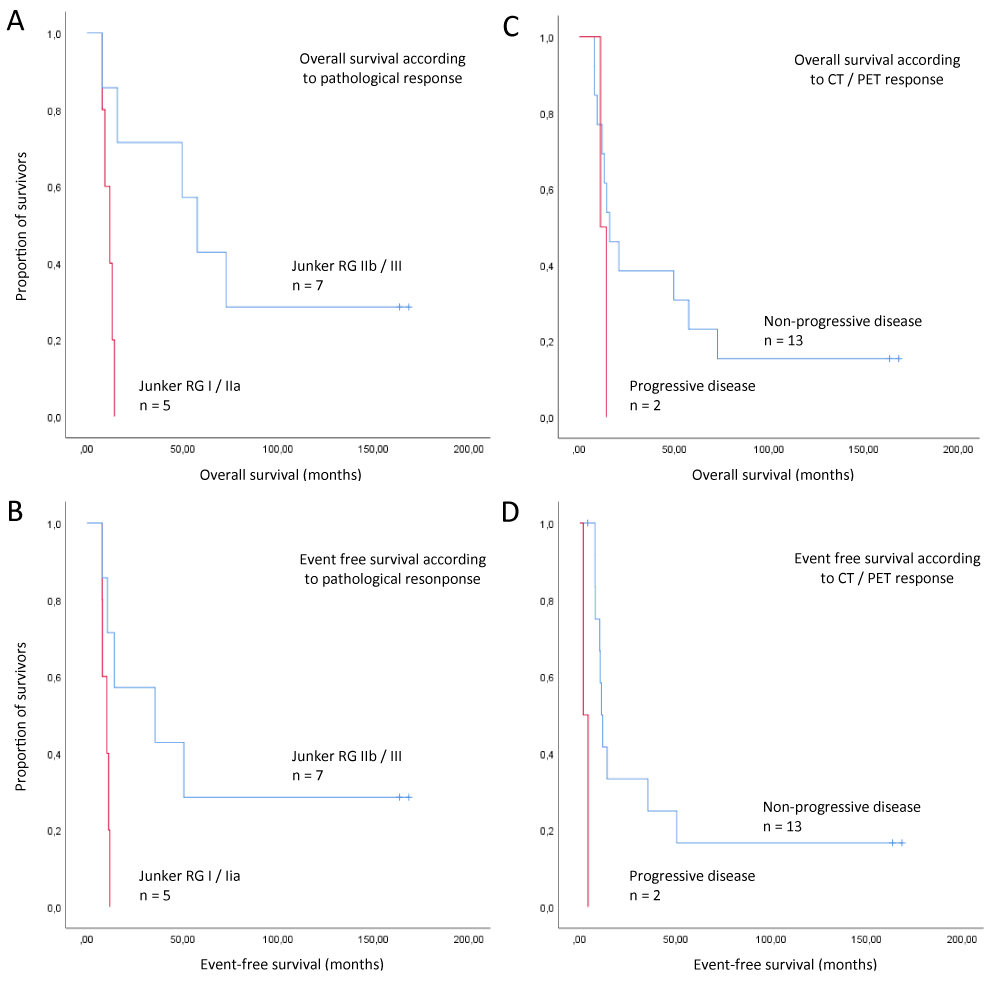
Kaplan-Meyer curves for overall survival and event-free survival according to CT / PET response are shown in figure 2.By July 2018, 10 of 12 patients (83.3%) having completed surgery had died. Median overall survival for patients according to Junker regression scale was 11.9 months (CI, 6.4 to 17.4) for patients with Junker RG I or IIa (n = 5) and 57.7 months (CI, 37.4 to 78.0) for RG score IIb / III (n = 7), p = 0,006 (log rank), level of significance only is descriptive. Kaplan-Meyer curves for overall survival dependent on Junker regression rate is shown in figure 2.
Figure 2: Overall survival analysis. Comparing patients with respect to pathological response after induction of E and GP + / - E, Junker regression grade RG IIb / III (major pathologic response) was associated with longer overall survival (A) and event free survival (B), n = 12 patients (4/16 patients without surgery). Comparison of response at CT / PET after Induction of E and GP + / - E showed longer overall survival (C) and event free survival (D) in patients with non-progressive disease, n = 15 patients (one patient with death before 2nd restaging). CT and PET were concordant in the matter of discrimination of progressive disease vs. non-progressive disease. These results only are descriptive.
How to identify the right patient for the right induction therapy? Before the description of EGFR mutations, clinical characteristics like never-smoking status, Asian ethnicity, adenocarcinoma and female gender were shown to be associated with response on EGFR directed therapy with TKIs like erlotinib[21]. Preclinical and clinical studies in lung cancer were set up to identify predictive markers for response to targeted therapy. The idea of the present study was to identify molecular patterns in patients by using state of the art affymetrix screening for molecular alterations and to correlate the results of the microarray analyses with pathological and clinical outcome of the study.
While unfortunately our study was not able, due to the slow recruitment and the insufficient number of patients to contribute to the detection of predictive biomarkers, the relevant predictive biomarkers have been identified for EGFR TKIs in the palliative setting: common mutations, such as Exon 19 deletions and Exon 21 mutations as well as uncommon mutations, especially of group I [21,22]. EGFR mutations have been identified to predict response to EGFR TKIs. Recently, the impact of co-mutations, such as TP53 and others has been appreciated. Also resistance mutations as EGFR T790M and MET amplification have been identified to confer acquired and sometimes innate resistance to EGFR TKIs.
While TKIs are standard of care in the palliative setting (stage IV), they still do not have a place in adjuvant or neoadjuvant setting, although these agents are associated in stage IV with an at least doubling of response rates. Almost no studies have been performed to investigate the value of TKIs in preoperative setting.
However, data have been generated in the adjuvant setting and after radio-chemotherapy in a consolidation setting. In the Southwest Oncology Group (SWOG) trial, reported by Kelly, et al., no benefit in unselected patients with stage IIIA and IIIB after definitive radio-chemotherapy and consolidation chemotherapy with docetaxel was observed in the group of patients treated with gefitinib in comparison to the observation group. Even worse, this population of unselected patients had an inferior overall survival and PFS if they received gefitinib as a consolidation therapy [23].
In the BR.19 study gefitinib as adjuvant treatment in completely resected NSCLC stage IB-IIIA patients did not yield to an improved overall survival or disease free survival, even in the group of patients with activating EGFR mutations [24].
A retrospective analysis by Janjigian, et al. [25] in a cohort of 167 completely resected NSCLC stages I-III with activating EGFR mutations compared the DFS and OS of patients receiving or not receiving TKI in the adjuvant setting. This non-randomized observational study revealed that patients receiving EGFR TKI (erlotinib or gefitinib) had a numerically improved DFS with no impact on overall survival.
A large randomized study to test the hypothesis of adjuvant TKI is the OSI RADIANT trial. No survival benefit was observed in the EGFR wild type patients as well as in the EGFR mutant patients, although a numerical benefit in PFS was seen in the latter group of patients [26].
A purely Chinese study was presented recently by Wu, et al., randomizing patients with stage II and IIIA (N1 and N2) with an activating EGFR mutation (Exon 19 or Exon 21) after curative surgery to gefitinib vs. cisplatin and vinorelbine. Primary endpoint was DFS, secondary endpoint were OS and other parameters. Although the primary endpoint was met with a 10.7 months benefit in median PFS, there was no overall survival benefit and there was also not a plateau of the PFS curve seen even after 4 years. Most likely a significant number of patients included in the trial did not have limited disease, as no mandatory PET-CT or magnetic resonance imaging (MRI) of the brain had been performed for staging. Therefore, these data did not change the treatment algorithm in early stage NSCLC with EGFR mutation [27]. Thus, as of yet EGFR-TKI have not been established in the adjuvant or consolidation setting even in EGFR mutant patients. Recently, Wu, et al. published data of the ADAURA trial, investigating adjuvant osimertinib vs. placebo in common EGFR mutant positive NSCLC stage IB to IIIA. Disease free survival was significantly longer in patients who received osimertinib. The overall survival data are still immature and have to be awaited before implementation into new recommendations [28].
The advantage of testing therapeutic modalities in the induction setting is to be able to treat patients “under sight” and also to have surgical material available for thorough analysis. Induction therapy has been established for a variety of cancers, the aim being to down-size and down-stage the tumor prior to surgery in order to improve outcome. While in the EGFR wild type population adjuvant therapy remains standard of care. In a meta-analyses induction therapy recently has been shown to be associated with a similar hazard ratio (HR) for PFS and OS as adjuvant therapy [1,11].
First trials of induction therapy date back in the early 90’s when Rosell, et al. [29] treated patients with stage IIIA with induction therapy, showing an improved survival for patients receiving induction therapy vs. observation. These trials were expanded to stage II to IIIB with various induction concepts: in summary all these trials show that patients with “sterilization” of the mediastinum have an excellent prognosis, especially if radiotherapy is not part of the induction therapy. Remission rates for chemotherapy with respect to pathologic complete response rate were in the range of 10% - 15% [16,10,30-32].
The potential of TKI in the curative induction setting has not been explored as of yet with the exception of very small studies or case reports. There is sparse data on the efficacy of TKI. A single case is reported with an exon 21 mutated tumor in a patient after induction therapy with gefitinib alone for 30 days with tumor response (assessed by PET-CT) and surgery with a near complete pathologic remission [33].
In a phase II study, 36 unselected patients with early lung cancer were treated with gefitinib for 28 days and histopathological features after gefitinib treatment were correlated with radiologic response. The only predictor of response was an EGFR mutation, since all 6 patients carrying those experienced at least some response and 50% achieved a PR after 28 days of treatment with gefitinib alone [34]. Molecular data, including resistance mutations were not studied by the group [35].
In a further phase II study by the group of Kris, et al. [36]. 50 patients with NSCLC stage I and II were enriched for EGFR mutations based on clinical criteria (never or light smoker and/or a component of broncho-alveolar carcinoma) and were treated for 21 days with gefitinib induction therapy. Response rate (> 25% tumor reduction) was 42%, 17 of the 21 responding patients had an EGFR mutation, 4 of 21 responding patients did not. Importantly, there were 4 patients with an EGFR mutation who did not respond in the induction setting to gefitinib (lesion size changes of -12%, -22%, -24%, and +14%). 2-year DFS was not improved in the group of EGFR mutated patients and was also not superior in the group of patients receiving adjuvant gefitinib compared with EGFR wild-type patients and those without adjuvant therapy.
Recently, the interest in combining EGFR-TKI with chemotherapy in patients carrying activating EGFR mutations has increased. Sequential administration of chemotherapy and TKI was first described in in vitro and in vivo models by the group of Gandara, et al. [37], suggesting that induction of apoptosis by chemotherapy and TKI was dependent by sequence of different type of systemic therapy.
The earliest signals of a potential clinical benefit of combining EGFR-TKI with chemotherapy came from the large phase III trial TRIBUTE. In this trial, unselected patients with metastatic NSCLC were treated with paclitaxel and carboplatin and randomized to receive erlotinib in the experimental arm. While the study was negative for overall survival as the primary endpoint, a molecular subgroup analysis in 29 patients with known EGFR mutation status was performed. It was shown that the presence of an activating EGFR mutation conferred better prognosis irrespectively of treatment in comparison to EGFR wild type patients. However, there was no clear difference in survival between the 15 patients with EGFR mutation receiving chemotherapy and erlotinib compared to the 14 patients with EGFR mutation receiving chemotherapy only. However, there was a numerical PFS benefit in the EGFR mutated group treated with chemotherapy and erlotinib compared to chemotherapy alone (p = 0,092). The response rate of the 29 EGFR mutated patients was 53% in the chemotherapy and erlotinib group (8/15) vs. 21% (3/14) in the chemotherapy group. The finding was not statistically significant, due to the low number of patients. Interestingly, the difference of CR and PR rate was statistically significantly different between the EGFR mutated patients and the EGFR wild type patients receiving chemotherapy and erlotinib (p < 0,01) indicating that EGFR TKI might be synergistic with chemotherapy for response induction [38].
Similar results were published for the INTACT 1 and 2 trials. Molecular analysis revealed that 13 of 18 EGFR-mutation carriers (72%) responded to chemotherapy plus gefitinib, compared to 84 of 152 mutation negative cases (55%), but this difference did not achieve statistical significance (p = 0.2). Only 5 patients with tumors carrying an activating EGFR mutation were available for response analysis, two of five (40%) responded to chemotherapy, this difference however was not statistically significant due to the low number of patients (p = 0,3) [39].
In one phase II study in unselected patients, erlotinib (150 mg/die p.o. days 2-16) was given in combination with docetaxel 70-75 mg/m2 d1 in patients with metastatic NSCLC. Response rate was 28,2% and DFS 4,1 months [40].
Similarly, erlotinib 250 mg/die p.o. and pemetrexed 500 mg/m2 every 22 days were feasible [41].
Combination therapy with paclitaxel and carboplatin was studied with intercalated erlotinib in a phase II trial by the Memorial Sloan Kettering Cancer Center (MSKCC) group in unselected stage IV NSCLC. Eighty-six unselected patients received paclitaxel 200 mg/m2 and carboplatin AUC 6,0 on day 3 with erlotinib 150 mg or 1500 mg on days 1 and 2, or paclitaxel 200 mg/m2 and carboplatin AUC 6,0 d1 and erlotinib 1500 mg d2 + 3. Primary endpoint was response rate. Response rates were 18% (5/28), 34% (10/29) and 28% (8/29), respectively. The most common grade 3 and 4 toxicities were neutropenia (39%), fatigue (15%), and anemia (12%). Grade 3 and 4 rash and diarrhea were uncommon [42]. Another randomized phase II trial in 181 predominantly Caucasian patients with advanced disease of NSCLC/adenocarcinoma compared erlotinib vs. erlotinib and paclitaxel and carboplatin followed by erlotinib in light or never smokers. In the EGFR mutant group, ORR was 70 vs. 73%, PFS was 14,1 vs. 17,2 months and OS 31,3 vs. 38,1 months [43].
The largest experience with sequential intercalated therapy has been generated in Asian patients: in the FAST-ACT trial, unselected patients with advanced non-small cell lung cancer received a backbone of chemotherapy consisting of gemcitabine 1250 mg/m2 d1+8 and carboplatin AUC 5,0 or cisplatin 75 mg/m2 d1. Erlotinib was administered after a lag period of 1 week after the last application of chemotherapy from days 15 to 28 [44]. Response rate was 35,5% for GP-erlotinib group versus 24,4% for GP-placebo group. PFS was significantly longer with GP-erlotinib than with GP-placebo (adjusted HR = 0,47; log-rank p ≥ 0,0002; median 29,4 vs. 23,4 weeks); this benefit was consistent across all clinical subgroups. The mutational status was known in only a subgroup of patients: 2/2 EGFR mutated patients receiving chemotherapy and erlotinib achieved PR. Three of 6 patients with EGFR mutation treated with chemotherapy alone achieved PR. In the group of never smokers, 24 patients receiving chemotherapy and erlotinib, 28 patients receiving chemotherapy only, the response rates were 45,8% vs. 32,1%. These phase II data suggested a potential synergistic effect of chemotherapy and intercalated erlotinib.
This concept was further studied in a phase III trial (FASTACT-2), in which 451 unselected Asian patients were recruited to receive the identical schedule as in the phase II trial. Biomarker data were available in 283 patients. The primary endpoint of the study was PFS, which was reached in the trial. PFS and OS in the group of patients with activating EGFR mutations (49 treated with chemotherapy + erlotinib, 48 chemotherapy and placebo) were significantly improved from 6.9 to 16.8 and 20.6 to 31.4 months, respectively, and with HR of 0,25 (p < 0,0001) and 0,48 (p = 0,0092), respectively. Objective response data were not available [45].
Five patients have recently been treated by the authors with TKI administration followed by chemotherapy and intercalated TKI in a neo-adjuvant setting. The treatment schedule proved to be feasible and led to a major pathologic response of mediastinal lymph nodes (Junker regression grade RG IIB - less than 10% tumor cells and signs of induction response) in 4 of 5 patients [15,46].
Systemic chemotherapy and administration of TKI employ different modes of action with regard to their anti-tumor activity. Combination of both treatments in a neo-adjuvant approach could potentially yield a valuable treatment option for EGFR mutant NSCLC patients promising superior tumor response and higher cure rate than current protocols. Response rate in the induction setting, especially mediastinal “sterilization” has been associated with excellent prognosis in stage II-III.
However, to date this therapeutic approach has not been investigated systematically and the best treatment schedule has not been established, since no formal scheduling studies have been performed in EGFR mutant patients. Actually the value of chemotherapy and TKI combination in a neo-adjuvant setting is still unclear and further molecular driven studies are warranted.
The concept of our present study was ambitious, as we wanted to identify predictive markers for response to EGFR TKI in the neo-adjuvant setting. Complexity of study related processes unfortunately was a barrier for acceptance of the study by physicians and accrual. Main obstacles first were the need of mediastinal evaluation obtaining tumor samples at baseline and immediate cryo-preservation of samples at baseline as well as during surgery. Additionally data arose for possible adverse effects of combining erlotinib or other tyrosine kinase inhibitors concomitant with chemotherapy as well as predictive data for response in patients with activating EGFR mutations [21]. Due to slow and poor recruitment and a high proportion of exclusion criteria for the main part of the study, the decision was taken to terminate the project early. This lead to the fact, that there was not enough power to detect any effect of any potential marker predicting response to EGFR TKI.
In our study we have identified one patient with an activating EGFR exon 19 mutation (adenocarcinoma of the lung, Caucasian male non-smoker). This patient responded with PR to erlotinib induction. PR also was seen in another patient with wild-type status for common activating EGFR mutations (exons 19 and 20), but insufficient material for testing of further molecular alterations. One patient with mutation in exon 18 of EGFR gene (squamous cell carcinoma of the lung, Caucasian male former heavy smoker) showed stable disease following erlotinib induction. All 3 patients responded with PR after platinum based chemotherapy. The patient with known exon 19 mutation had recurrent disease after 50,6 months and finally got erlotinib for about further 10 months with good clinical response until death after 72,8 months since study entry. The patient with known exon 18 mutation (P694L) had recurrent disease at 24,9 months and showed clinical benefit to reapplied erlotinib for further 13 months and finally died 49,7 months after study entry. So far, response data to EGFR TKIs in patients with P694L mutation never have been published before. Detailed clinical features of our patient with exon 18 P694L mutation have been described in another context [47]. P694L mutation (exon 18) is a rare event. Ming, et al. in a retrospective analysis of BR.21 study identified in total 45 mutations in 40 patients, only 3 mutations were found in exon 18, herein once P694L as a novel mutation [48]. In a Chinese population of 354 screened patients with lung cancer 48% were EGFR mutation positive, only 1 patient with P694L alteration was identified (0,3%), but without any data on response to TKI and outcome [49].
In our study there were also two female patients with a KRAS mutation, both are still alive over more than 163 and 168 months, respectively, after first diagnosis of lung cancer. Zhang, et al. concluded in a meta-analysis of studies with resected NSCLC patients with EGFR or KRAS mutations that EGFR mutations were a benign prognostic factor for DFS and OS of resected NSCLC and in addition, that KRAS mutations were a poor prognostic factor for DFS and OS in patients with NSCLC after surgery [50]. As a limitation, this meta-analysis disregarded the effect of neo-adjuvant or adjuvant systemic approach or consequent therapies potentially targeting molecular alterations. However, it is beyond controversy, that patients with activating EGFR mutation (and targeted therapy) achieve a better outcome. The only patient with exon 19 deletion in our study reached a 7 years-survival and the one with exon 18 mutation achieved 4 years.
In contrast to the meta-analysis, in our study longest survival of more than 13 years only was seen in two KRAS positive patients. The small number of patients and narrow investigated molecular profile do not allow to make any definitive conclusion, but could advise not to exclude apparently prognostic negative subgroups from innovative approaches. Unfortunately, due to the small number of patients it was further not possible to analyze more in detail the molecular pattern in the context of clinical outcome.
Data have been generated to suggest that patients with major pathologic regression (vital tumor cells below 10%) have an improved prognosis [3]. This corresponds with survival data in this trial with an improvement of median overall survival by factor 4.8 for patients with major vs. minor or missing pathologic response following induction therapy. In contrast, the meta-analysis of Jeremić et al. could not identify any treatment-related predictive or prognostic factors for selecting surgery in the treatment of patients with stage IIIA/pN2 NSCLC. This result is limited by the fact, that factors, such as the degree of tumor regression, status of surgical margins, evaluation of response before surgery, or post-induction tumor status (ypT), were evaluated only in slightly more than 40% of all available studies [51].
There is an unmet medical need to improve outcome in limited stage NSCLC. It is known that the mediastinal down-staging in N2/N3 disease is associated with improved survival specifically that the grade of regression correlates with disease free survival and overall survival.
Although TKIs like EGFR TKI and ALK TKI are associated with remission rates in the palliative setting that are at least doubled in comparison to chemotherapy, these highly potential agents have not been rigorously studied in the setting where a high remission rate is crucial. Therefore, there is an urgent need to design trials in the neo-adjuvant setting that address the question of whether EGFR targeted TKI and potentially other TKI might have a place in the induction setting. With the progress that has been made in the rapid and comprehensive detection of molecular alterations, these trials might be easier to perform nowadays as compared to 10 years ago.
Are such trials possible in the Caucasian population? Most likely not. Although testing has been adopted in Europe and the USA broadly for non-squamous NSCLC in stage IV, testing is currently not done on a regular basis in the early setting. Furthermore, it takes 100 patients to identify 10 patients with EGFR mutations in the Caucasian world. Also, the Arbeitsgemeinschaft Internistische Onkologie (AIO) group has tried to perform an induction trial in stage II and III NSCLC with EGFR activating mutation. While the percentage of EGFR mutated patients in stage IV is between 10% to 15%, in this trial the rate of EGFR mutated NSCLC was at 5%. Also this trial was stopped prematurely because of slow recruitment. Most likely these trials would have to be performed in Asia, where the incidence of EGFR mutated NSCLC is about 5 times higher as in the Caucasian population. Strict staging procedures including PET-CT, MRI of the brain as well as pathologic mediastinal staging are required to generate reliable data. Most likely a phase II trial with pathologic remission as primary endpoint would suffice to generate a signal to decide whether a phase III trial would be necessary. Given a pathologic CR (pCR) rate of between 10% to 15% after induction chemotherapy alone, a pCR rate that would be tripled would most likely be accepted as a leap innovation.
Recently, induction studies with immune-checkpoint-inhibitors have been performed with pathologic remission as secondary [3,5] or primary endpoint [4]. Major pathologic response (MPR), defined as < = 10% viable tumor cells in resected primary tumors, was reached in 9/21 patients (43%) including 3/21 patients (14%) with pathological complete responses (pCR) [5] and reached up to 76% for MPR (intent to treat population, ITT), and 45% for pCR (ITT), respectively. Data with PD-1 inhibitor monotherapy or in combination with ipilimumab achieved a MPR rate of 25% (11/44 patients) and a pCR of 18% (8/44 patients) [4].
Remarkably, there was no patient with pCR in our study, whereas MPR rate was 58% (combined for primary tumors and lymph nodes, 7/12 patients). Future induction trials might contain non-gemcitabine platinum doublet therapy combined with immunotherapy to reach the highest degree of pathologic remission. Radiologic based response rate in stage IV disease showed best results for combination of platinum based chemotherapy with pembrolizumab of about 48% irrespective of PD-L1 status [52] and for pembrolizumab monotherapy of about 45% in patients with PD-L1 status of 50% and more [53,54]. However, patients with molecular defined tumors like EGFR or ALK alterated NSCLC did not show benefit from addition of immunotherapy to a platinum based doublet, but combination of VEGF-inhibition with a triple chemo-immun combination yielded in a potential benefit after utilizing a targeted approach [52,55]. New approaches of induction therapy in molecular defined tumors might integrate chemo-immunotherapy, but also might consider adding targeted therapies.
What can be learned from our early terminated clinical study?
In this patient population, neo-adjuvant single agent and combination of TKI with platinum based combination therapy was feasible. No new toxicity signals were recorded. The stepwise approach of starting chemotherapy after TKI therapy assured to induce remission also in patients with stable or progressive disease following TKI. Also, no increased lung toxicity was seen in patients that received post-surgery mediastinal irradiation. The combination of TKI and chemotherapy was well tolerated, surgical removal was feasible after induction therapy.
In conclusion, our study was, in an era before molecular mechanisms in NSCLC were known, an attempt to identify specific subgroups based on molecular alterations that was overtaken by molecular pathology and pre-specified subgroups in following trials.
Clinical practice points
In locally advanced lung cancer, patients are treated with a curative intent by a multimodal approach with surgery, systemic therapy and in some cases radiotherapy or a definitive concomitant radio- and chemotherapy procedure in order to achieve long term disease free survival as well as overall survival. Surrogate endpoints like pathologic or radiologic remission are of high interest predicting long term survival without recurrent disease. We present an induction study in locally advanced NSCLC, which was started in 2004 using TKI and chemotherapy for all comer patients. The study was started before knowledge of EGFR mutations and intended to identify patients, who would respond to EGFR TKI. Two patients with EGFR mutations were identified, that responded very well, but finally relapsed. Overall, the concept of TKI + chemotherapy was feasible, major pathologic response was predictive of disease free survival and overall survival and surprisingly the only long term survivors were KRAS mutant positive NSCLC. Although this trial stopped early, it nicely demonstrates the potential of systematic induction therapy trials for the development of new induction strategies.
We thank Hoffmann-La Roche for providing financial support to this trial. We also thank to our patients and investigators for their support.
Authors’ disclosures of potential conflicts of interests
This study has been supported by Hoffmann-La Roche.
TRO received grants as an advisor or speaker from: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Hoffmann-La Roche, Merck Sharp and Dohme, Novartis Pharma, Pfizer, Takeda Oncology, Tesaro. TRO received travel support from AstraZeneca.
WK received grants as an advisor or speaker from: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Hoffmann-La Roche, Eli Lilly, Merck Sharp and Dohme, Novartis Pharma.
MT received grants as an advisor or speaker from: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Hoffmann-La Roche, Merck Sharp and Dohme, Novartis Pharma, Pfizer, Takeda Oncology.
CP, BCD, BH, CT, ES, KT, MF and SHPW have no conflicts of interests to declare.
FG received funding from: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Merck Sharp and Dohme, Novartis Pharma, Pfizer, Hoffmann-La Roche, Takeda, Siemens. FG received grants as an advisor or speaker from: Abbvie, Amgen, Ariad, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Hoffmann-La Roche, Merck Sharp and Dohme, Novartis Pharma, Pfizer, Siemens, Takeda Oncology, Tesaro.
Authors’ contributions
TRO Conceptualization, Methodology, Software, Resources, Validation, Formal analysis, Investigation, Project administration, Data curation, Writing – Original Draft, Review & Editing, Visualization
SHPW Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – Review & Editing
BD Investigation, Writing – Review & Editing
WK Investigation, Writing – Review & Editing
KT Investigation, Writing – Review & Editing
BH Investigation, Formal analysis, Writing – Review & Editing
CP Investigation, Formal analysis, Writing – Review & Editing
MF Investigation, Formal analysis, Writing – Review & Editing
MT Investigation, Formal analysis, Writing – Review & Editing
CT Resources, Project administration Writing – Review & Editing
ES Resources, Project administration, Writing – Review & Editing
FG Conceptualization, Methodology, Investigation, Validation, Resources, Formal analysis, Funding acquisition, Writing – Original Draft, Review & Editing.
All authors provided final approval of the version to be published and provided agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
- NSCLC Meta-analyses Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010; 375: 1267–1277. PubMed: https://pubmed.ncbi.nlm.nih.gov/20338627/
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018; 379: 2342–2350. PubMed: https://pubmed.ncbi.nlm.nih.gov/30280658/
- Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020; 21: 1413–1422. PubMed: https://pubmed.ncbi.nlm.nih.gov/32979984/
- Cascone T, William WN, Weissferdt A, Lin HY, Leung CH, et al. Neoadjuvant nivolumab (N) or nivolumab plus ipilimumab (NI) for resectable non-small cell lung cancer (NSCLC): Clinical and correlative results from the NEOSTAR study. J Clin Oncol. 2019; 37(15_suppl): 8504.
- Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med. 2018; 378: 1976–1986. PubMed: https://pubmed.ncbi.nlm.nih.gov/29658848/
- Roth JA, Atkinson EN, Fossella F, Komaki R, Bernadette Ryan M, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer. 1998; 21: 1–6. PubMed: https://pubmed.ncbi.nlm.nih.gov/9792048/
- Roth JA, Fossella F, Komaki R, Ryan MB, Putnam JB, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst. 1994; 86: 673–680. PubMed: https://pubmed.ncbi.nlm.nih.gov/8158698/
- Rosell R, Gomez-Codina J, Camps C, Maestre J, Padille J, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. New Engl J Med.1994; 330: 153–158. PubMed: https://pubmed.ncbi.nlm.nih.gov/8043059/
- Pass HI, Pogrebniak HW, Steinberg SM, Mulshine J, Minna J. Randomized trial of neoadjuvant therapy for lung cancer: interim analysis. Ann Thorac Surg. 1992; 53: 992–998. https://pubmed.ncbi.nlm.nih.gov/1317697/
- Thomas M, Rube C, Hoffknecht P, Macha HN, Freitag L, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol. 2008; 9: 636–648. PubMed: https://pubmed.ncbi.nlm.nih.gov/18583190/
- NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014; 383: 1561–1571. PubMed: https://pubmed.ncbi.nlm.nih.gov/24576776/
- Horita N, Miyazawa N, Morita S, Kojima R, Kimura N, et al. Preoperative Chemotherapy Is Effective for Stage III Resectable Non–Small-Cell Lung Cancer: Metaanalysis of 16 Trials. Clin Lung Cancer. 2013; 14: 488–494. PubMed: https://pubmed.ncbi.nlm.nih.gov/23664722/
- Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008; 26: 3552–3559. PubMed: https://pubmed.ncbi.nlm.nih.gov/18506026/
- Felip E, Rosell R, Maestre JA, Rodríguez-Paniagua JM, Morán T, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol. 2010; 28: 3138–3145. PubMed: https://pubmed.ncbi.nlm.nih.gov/20516435/
- Junker K, Langner K, Klinke F, Bosse U, Thomas M. Grading of tumor regression in non-small cell lung cancer: morphology and prognosis. Chest. 2001; 120: 1584–1591. PubMed: https://pubmed.ncbi.nlm.nih.gov/11713138/
- Betticher DC, Hsu Schmitz SF, Totsch M, Hansen E, Joss C, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol. 2003; 21: 1752–1759. PubMed: https://pubmed.ncbi.nlm.nih.gov/12721251/
- Cerfolio RJ, Bryant AS, Winokur TS, Ohja B, Bartolucci AA. Repeat FDG-PET after neoadjuvant therapy is a predictor of pathologic response in patients with non-small cell lung cancer. Ann Thorac Surg. 2004; 78:1903-1909. PubMed: https://pubmed.ncbi.nlm.nih.gov/15560998/
- Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007; 25: 1545–1552. PubMed: https://pubmed.ncbi.nlm.nih.gov/17442998/
- Hidalgo M, Siu LL, Nemunaitis J, Rizzo J, Hammond LA, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001; 19: 3267–3279. PubMed: https://pubmed.ncbi.nlm.nih.gov/11432895/
- Ratain MJ, George CM, Janisch L, et al. Phase I trial of erlotinib (OSI-774) in combination with gemcitabine (G) and cisplatin (P) in patients with advanced solid tumors. Proc Am Soc Clin Oncol. 2002; 21: 76b.
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. New Engl J Med. 2004; 350: 2129–2139. PubMed: https://pubmed.ncbi.nlm.nih.gov/15118073/
- Yang JCH, Shih JY, Su WC, Hsia TC, Tsai CM, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol. 2012; 13: 539–548. PubMed: https://pubmed.ncbi.nlm.nih.gov/22452895/
- Kelly K, Chansky K, Gaspar LE, Albain KS, Jett J, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008; 26: 2450–2456. PubMed: https://pubmed.ncbi.nlm.nih.gov/18378568/
- Goss GD, O'Callaghan C, Lorimer I, Tsao MS, Masters GA, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013; 31: 3320–3326. PubMed: https://pubmed.ncbi.nlm.nih.gov/23980091/
- Janjigian YY, Park BJ, Zakowski MF, Ladanyi M, Pao W, et al. Impact on disease-free survival of adjuvant erlotinib or gefitinib in patients with resected lung adenocarcinomas that harbor EGFR mutations. J Thorac Oncol. 2011; 6: 569–575. PubMed: https://pubmed.ncbi.nlm.nih.gov/21150674/
- Kelly K, Altorki NK, Eberhardt WEE, O'Brien MER, Spigel DR, et al. Adjuvant Erlotinib Versus Placebo in Patients With Stage IB-IIIA Non-Small-Cell Lung Cancer (RADIANT): A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2015; 33: 4007–4014. PubMed: https://pubmed.ncbi.nlm.nih.gov/26324372/
- Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. 2018; 19: 139–148. PubMed: https://pubmed.ncbi.nlm.nih.gov/29174310/
- Wu YL, Tsuboi M, He J, John T, Grohe C, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med. 2020; 383: 1711–1723. PubMed: https://pubmed.ncbi.nlm.nih.gov/32955177/
- Rosell R, Maestre J, Font A, Moreno I, Molina F, et al. A randomized trial of mitomycin/ifosfamide/cisplatin preoperative chemotherapy plus surgery versus surgery alone in stage IIIA non-small cell lung cancer. Semin Oncol. 1994; 21(3 Suppl 4): 28–33. PubMed: https://pubmed.ncbi.nlm.nih.gov/8209274/
- Daly BDT, Cerfolio RJ, Krasna MJ. Role of surgery following induction therapy for stage III non-small cell lung cancer. Surg Oncol Clin N Am. 2011; 20: 721–732. PubMed: https://pubmed.ncbi.nlm.nih.gov/21986268/
- Herse B, Dalichau H, Wormann B, Hemmerlein B, Schmidberger H, et al. Induction combination chemotherapy with docetaxel and carboplatin in advanced non-small-cell lung cancer. Thorac Cardiovasc Surg. 1998; 46: 298–302. PubMed: https://pubmed.ncbi.nlm.nih.gov/27517545/
- Poettgen C, Theegarten D, Eberhardt W, Levegruen S, Gauler T, et al. Correlation of PET/CT findings and histopathology after neoadjuvant therapy in non-small cell lung cancer. Oncology. 2007; 73: 316–323. PubMed: https://pubmed.ncbi.nlm.nih.gov/18497503/
- Liu M, Jiang G, He W, Zhang P, Song N. Surgical resection of locally advanced pulmonary adenocarcinoma after gefitinib therapy. Ann Thorac Surg. 2011; 92: e11-2. PubMed: https://pubmed.ncbi.nlm.nih.gov/21718818/
- Lara-Guerra H, Waddell TK, Salvarrey MA, Joshua AM, Chung CT, et al. Phase II study of preoperative gefitinib in clinical stage I non-small-cell lung cancer. J Clin Oncol. 2009; 27: 6229–6236. PubMed: https://pubmed.ncbi.nlm.nih.gov/19884551/
- Lara-Guerra H, Chung CT, Schwock J, Pintilie M, Hwang DM, et al. Histopathological and immunohistochemical features associated with clinical response to neoadjuvant gefitinib therapy in early stage non-small cell lung cancer. Lung Cancer. 2012; 76: 235–241. PubMed: https://pubmed.ncbi.nlm.nih.gov/22112291/
- Rizvi NA, Rusch V, Pao W, Chaft JE, Ladanyi M, et al. Molecular characteristics predict clinical outcomes: prospective trial correlating response to the EGFR tyrosine kinase inhibitor gefitinib with the presence of sensitizing mutations in the tyrosine binding domain of the EGFR gene. Clin Cancer Res. 2011; 17: 3500–3506. PubMed: https://pubmed.ncbi.nlm.nih.gov/21558399/
- Gandara D, Narayan S, Lara PN, JR, Goldberg Z, Davies A, et al. Integration of novel therapeutics into combined modality therapy of locally advanced non-small cell lung cancer. Clin Cancer Res. 2005; 11: 5057s-5062s. PubMed: https://pubmed.ncbi.nlm.nih.gov/16000614/
- Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005; 23: 5900–5999. PubMed: https://pubmed.ncbi.nlm.nih.gov/16043828/
- Bell DW, Lynch TJ, Haserlat SM, Harris PL, Okimoto RA, et al. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol. 2005; 23: 8081–8092. PubMed: https://pubmed.ncbi.nlm.nih.gov/16204011/
- Sangha R, Davies AM, Lara PN, JR, Mack PC, Beckett LA, et al. Intercalated erlotinib-docetaxel dosing schedules designed to achieve pharmacodynamic separation: results of a phase I/II trial. J Thorac Oncol. 2011; 6: 2112–2119. PubMed: https://pubmed.ncbi.nlm.nih.gov/21892109/
- Davies AM, Ho C, Beckett L, Lau D, Scudder SA, et al. Intermittent erlotinib in combination with pemetrexed: phase I schedules designed to achieve pharmacodynamic separation. J Thorac Oncol. 2009; 4: 862–868. PubMed: https://pubmed.ncbi.nlm.nih.gov/19494788/
- Riely GJ, Rizvi NA, Kris MG, Milton DT, Solit DB, et al. Randomized phase II study of pulse erlotinib before or after carboplatin and paclitaxel in current or former smokers with advanced non-small-cell lung cancer. J Clin Oncol. 2009; 27: 264–270. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2645610/
- Janne PA, Wang X, Socinski MA, Crawford J, Stinchcombe TE, Gu L et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol. 2012; 30: 2063–2069. PubMed: https://pubmed.ncbi.nlm.nih.gov/22547605/
- Mok TSK, Wu YL, Yu CJ, Zhou C, Chen YM, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2009; 27: 5080–5087. PubMed: https://pubmed.ncbi.nlm.nih.gov/19738125/
- Wu YL, Lee JS, Thongprasert S, Yu CJ, Zhang L, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol. 2013; 14: 777–786. PubMed: https://pubmed.ncbi.nlm.nih.gov/23782814/
- Griesinger F, Roeper J, Netchaeva M, Lueers AC, Falk M, et al. Intercalated EGFR TKI and chemotherapy induction therapy in EGFR mt+ NSCLC stage IIIA and IIIB: report of 5 cases. J Clin Oncol. 2016; 34(15_suppl): e20059-e20059.
- Overbeck TR, Griesinger F. Two cases of psoriasis responding to erlotinib: time to revisiting inhibition of epidermal growth factor receptor in psoriasis therapy? Dermatology (Basel). 2012; 225: 179–182. PubMed: https://pubmed.ncbi.nlm.nih.gov/23095682/
- Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, et al. Erlotinib in Lung Cancer — Molecular and Clinical Predictors of Outcome. New Engl J Med. 2005; 353: 133–144. PubMed: https://pubmed.ncbi.nlm.nih.gov/16014883/
- Quan X, Gao H, Wang Z, Li J, Zhao W, et al. Epidermal growth factor receptor somatic mutation analysis in 354 Chinese patients with non-small cell lung cancer. Oncol Lett. 2018; 15: 2131–2138. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5776883/
- Zhang SM, Zhu QG, Ding XX, Lin S, Zhao J, et al. Prognostic value of EGFR and KRAS in resected non-small cell lung cancer: a systematic review and meta-analysis. Cancer Manag Res. 2018; 10: 3393–3404. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6138965/
- Jeremić B, Casas F, Dubinsky P, Gomez-Caamano A, Čihorić N, et al. Treatment-Related Predictive and Prognostic Factors in Trimodality Approach in Stage IIIA/N2 Non-Small Cell Lung Cancer. Frontiers Oncol. 2018; 8: 30. PubMed: https://pubmed.ncbi.nlm.nih.gov/29527511/
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018; 378: 2078–2092. PubMed: https://pubmed.ncbi.nlm.nih.gov/29658856/
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol. 2019; 37: 537–546. PubMed: https://pubmed.ncbi.nlm.nih.gov/30620668/
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016; 375: 1823–1833. PubMed: https://pubmed.ncbi.nlm.nih.gov/27718847/
- Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018; 378: 2288–2301. PubMed: https://pubmed.ncbi.nlm.nih.gov/29863955/