More Information
Submitted: 01 September 2020 | Approved: 15 September 2020 | Published: 16 September 2020
How to cite this article: Kasongo ANW, Mukuku O, Kanteng GA, Shongo MY, Mutombo AJ, et al. General practitioners’ knowledge, attitudes and practices on antibiotic prescribing for acute respiratory infections in children in Lubumbashi, Democratic Republic of Congo. J Pulmonol Respir Res. 2020; 4: 011-017.
DOI: 10.29328/journal.jprr.1001015
ORCiD: orcid.org/0000-0001-6902-7023
Copyright License: © 2020 Kasongo ANW, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Respiratory tract infections; Child; Anti-bacterial agents; Evidence-based practice
General practitioners’ knowledge, attitudes and practices on antibiotic prescribing for acute respiratory infections in children in Lubumbashi, Democratic Republic of Congo
Aubin Ndjadi Wembonyama Kasongo1,4, Olivier Mukuku2*, Gray A-Wakamb Kanteng1, Mick Ya-Pongombo Shongo1, André Kabamba Mutombo3, Albert Mwembo-A-Nkoy Tambwe4, Dieudonné Tshikwej Ngwej1, Stanis Okitotsho Wembonyama1,4 and Oscar Numbi Luboya1,4
1Department of Pediatrics, University of Lubumbashi, Democratic Republic of Congo
2Higher Institute of Medical Techniques of Lubumbashi, Democratic Republic of Congo
3Department of Pediatrics Official University of Mbuji-Mayi, Democratic Republic of Congo
4School of Public Health, University of Lubumbashi, Democratic Republic of Congo
*Address for Correspondence: Olivier Mukuku, Higher Institute of Medical Techniques of Lubumbashi, Democratic Republic of Congo, Tel: +243 997925649; Email: [email protected]
Objective: To assess the knowledge, attitudes and practices declared among general practitioners (GPs) concerning the use of antibiotics for the treatment of ARI in children under 5 years in Lubumbashi.
Methods: A cross-sectional survey was conducted to assess the level of knowledge, attitude and practices concerning antibiotic prescribing among 67 GPs working in the pediatric setting in various health structures in Lubumbashi city, in the Democratic Republic of Congo. Data were collected from April 1st to June 30th, 2020.
Results: GPs had limited knowledge about antibiotic prescriptions (mean of 46% correct answers to 8 questions). Although they are generally concerned about antibiotic resistance (mean ± SD = 0.50 ± 0.68), and are unwilling to submit to pressure to prescribe antibiotics to meet patient demands and expectations (mean ± SD = –1.78 ± 0.31) and the requirements to prescribe antibiotics for fear of losing patients (mean ± SD = –1.67 ± 0.47), there was a lack of motivation to change prescribing practices (mean ± SD = −0.37 ± 0.94) and strong agreement that they themselves should take responsibility for tackling antibiotic resistance (mean ± SD = 1.24 ± 0.74). Multiple linear regression results showed that higher knowledge scores were associated with less avoidance of responsibility when prescribing antibiotics (β = 0.919; p = 0.000).
Conclusion: To curb the over-prescription of antibiotics, it is not enough to improve knowledge in itself. The lack of motivation of physicians to change must be addressed through a systematic approach. These data show the need for interventions that support the rational prescribing of antibiotics.
Acute respiratory infections (ARIs) include all conditions of the respiratory tract, ranging from the common cold to lung infections. They are among the leading causes of infant morbidity and mortality in developing countries, accounting for 4.3 million of the 13 million infant deaths recorded each year worldwide [1]. This has led to a renewed interest in reducing morbidity and mortality associated with ARIs, which are mainly due to pneumonia [2].
Since most ARIs are caused by viruses, antibiotics have limited therapeutic value and should only be prescribed if a bacterial origin is suspected, documented and justified [2]. Studies report high rates of prescribing antibiotics for ARI in young children [3,4]. Contributing factors include physician diagnostic uncertainty, parents ‘expectation of receiving antibiotics, and physicians’ perceptions of parental satisfaction at the consultation [5,6].
The widespread use of antibiotics, whether appropriate or inappropriate, has led to the emergence and spread of bacteria resistant to these molecules [7]. Resistance to antibiotics used among respiratory pathogens has become a common clinical problem [8]. Antibiotic resistance has emerged as one of the most serious global health development issues, threatening the optimal ability to treat common infectious diseases [9]. The irrational use of antibiotics has been observed in various parts of the world among those involved in prescribing, selling and taking drugs [10,11]. Physicians play an essential role in the global campaign against antibiotic resistance, through unspecified and unencrypted prescriptions [12,13]. It is important to understand how general practitioners (GPs) prescribe these drugs. GPs can defy good practice guidelines and prescribe antibiotics to meet patient expectations or avoid potential patient confrontations and complaints [12]. This process could involve other tradeoffs since most prescribers are aware of the side effects of overuse of antibiotics. Few studies have been conducted on the knowledge, attitudes and practices of health care providers regarding the correct use of antibiotics, especially in low-income settings.
In the Democratic Republic of Congo (DRC), symptoms of ARIs are the most common reason for seeking health care for children. Recommendations for the rational use of antibiotics for the treatment of ARIs in children have been formulated internationally and adapted into national guidelines [14,15]. The Integrated Management of Childhood Illnesses (IMCI) program has been implemented to address major child health problems in the DRC, including ARIs. Although there is this manual on IMCI, the current management of ARI in children in the DRC, particularly in general practice, is unclear. The knowledge, attitudes and practices of physicians in Lubumbashi on antibiotic prescribing for ARIs in children is limited.
The aim of this study was to assess the knowledge, attitudes and practices declared among GPs regarding antibiotic prescribing for the treatment of ARIs in children under 5 years in Lubumbashi. Understanding these factors can help develop appropriate intervention strategies to improve antibiotic prescribing and dispensing practices for ARIs and reduce the spread of antibiotic resistance.
Study design and settings
This study was conducted in Lubumbashi, in Haut-Katanga province. The city of Lubumbashi is located in the south-east of the DRC and has a population of nearly 4 million. This study focused on health centers (HC), general referral hospitals (GRH), private clinics (PC) as well as University clinics (UC).
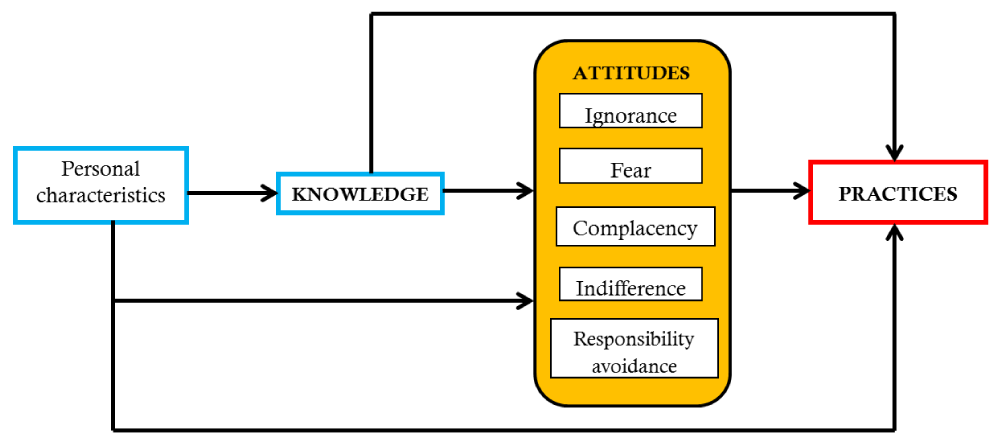
The theoretical model proposed by Teixeira (Figure 1) was adapted for this study, developed on the basis of the theory of knowledge, attitudes and practices (KAP) [16] and revised on the basis of several systematic reviews [17,18]. Knowledge was tested using 8 standard questions [12,19,20] and attitudes were classified into 5 aspects (Figure 1) [17,18]:
Figure 1: Theoretical framework of knowledge, attitudes and practices regarding antibiotic prescribing.
Ignorance: a lack of concern about antibiotic resistance resulting from over-prescribing of antibiotics;
Fear: prescribing antibiotics for fear of losing patients or losing in potential conflicts with patients;
Complacency: prescribing antibiotics to meet patient demands and expectations;
Responsibility avoidance: a belief that others (patients, governments and other professionals) are responsible for the problem of antibiotic resistance; and
Indifference: a lack of motivation to change antibiotic prescribing practices.
Data collection and quality control instrument
A 47-item questionnaire was developed to measure the knowledge, attitudes, practices and personal characteristics of physicians associated with antibiotic prescribing. Knowledge was measured using 8 questions, asking respondents to make a judgment on when antibiotics (or a type of antibiotic) should or should not be prescribed. Some questions have been borrowed from previous studies [12,20,21]. Three additional questions were added: two regarding antibiotic prescriptions for ARIs; and one on WHO recommendations for the use of antibiotics in primary health care. The questionnaire was closed and self-administered with for each question 4 or 5 assertions of which only one had been considered correct. Respondents were also given the option to choose “Don’t know”.
Attitudes were measured using 27 items on a 5-point Likert scale, with each subscale containing at least 3 items. These elements were adapted from 2 validated instruments, taking into account the results of several literature reviews [12]. Regarding practices, the first 2 subscales were measured using 3 items each, drawing on the actions of “wanting, waiting and planning”, respectively, along a 5-point Likert scale, while the last used a single element estimating the number of patients with ARIs (out of 10) for whom antibiotics could be prescribed [12].
Personal characteristics recorded in this study included age, gender, type of health facility where respondents worked, years of clinical practice, and training related to antibiotics.
A pilot study was carried out with 10 physicians from 3 health structures. Participants were invited to complete the questionnaire and provide comments on the relevance, clarity and difficulty of the elements of the questionnaire. This has led to the adaptation, revision, addition or deletion of certain elements of the survey tool.
Sampling and data collection
This study was a survey consisting of interviewing general practitioners working in the pediatric context of different HC, GRH, UC and PC in the city of Lubumbashi chosen by random selection. In each health facility surveyed, general practitioners (if any) present during the survey period were approached and invited to participate in the survey. Data were collected from April 1st to June 30th, 2020. Each facility was visited by a pair of trained investigators. The recruited investigators received intensive training, covering the context of the current survey, detailed interpretation of the survey instrument and a survey simulation test.
Finally, a total of 5 investigators were recruited. GPs working in administrative departments and those whose tasks rarely involved prescriptions for antibiotics were excluded from the survey. The investigators explained the purpose and procedure of the study and obtained the informed written consent of each respondent before asking them to complete the questionnaire. On average, the investigation lasted 10 to 15 minutes. The entire returned questionnaire was examined by the investigators, the missing elements (if any) being modified through additional on-site interviews. A total of 100 questionnaires were distributed and 75 were returned. Of the returned questionnaires, 67 contained no missing items and were included for further analysis. This represented an effective response rate of 67%.
Data analysis
The percentages of respondents who gave a correct answer to each question and the total number of correct answers per participant were calculated. Each attitude item was coded to a 5-point Likert scale, with a negative score indicating disagreement and a positive score indicating agreement with relevant evidence-based good practice. Scores in the same subdomain were added and averaged (ranging from -2 to 2).
Prescribing practices were assessed using 3 indicators: percentage of prescriptions containing antibiotics for ARIs, mean score for antibiotic prescribing efforts, and mean score for efforts to reduce antibiotic prescriptions. The latter two were coded in the same way as the attitude measures, with a negative score indicating refusal and a positive score indicating favorable to reducing antibiotic prescriptions (ranging from -2 to 2).
Data were analyzed using descriptive statistics. For continuous data, the mean with standard deviation was used. For categorical data, frequency and percentages were calculated. Differences between respondents in their knowledge, attitudes, and practices towards antibiotic prescriptions were examined using the Chi-square test (or Fisher’s exact test when recommended) as well as the parametric ANOVA test for the comparison of score means. Multiple linear regressions were used to examine correlations between level of knowledge and personal characteristics, attitudes and practices of antibiotic prescribing. Statistical analyzes were performed using STATA (version 12.0). A value of p < 0.05 was considered statistically significant.
Ethical considerations
This study was approved by the medical ethics committee of the University of Lubumbashi (Approval number:
UNILU/CEM/172/2019). The informed consent of all participants was requested after the initiation of the study. Confidentiality was ensured and participants were informed that they had the right to withdraw from the study at any time without prior explanation from them.
Respondent personal characteristics
The 67 respondents had a mean age of 35.96 ± 4.09 years and most (62.69%) were men. More than half (59.70%) of the respondents worked in a PC. On average, respondents had 6 years of clinical experience and only nearly 20% had received antibiotic training in the past two years prior to the survey (Table 1).
| Table 1: Personal characteristics of respondents | ||
| Variable | Mean ± SD | N (%) |
| Age (years) | 35.96 ± 4.09 | |
| Gender | ||
| Female | 25 (37.31) | |
| Male | 42 (62.69) | |
| Type of health facility | ||
| Private clinic | 40 (59.70) | |
| University clinics | 16 (23.88) | |
| GRH / HC | 11 (16.42) | |
| Clinical experience (years) | 6.31 ± 3.58 | |
| Had received antibiotic training in the past two years | ||
| No | 54 (80.60) | |
| Yes | 13 (19.40) | |
| GRH: General Reference Hospital; HC: Health Center; SD: standard deviation; N: number; %: pourcentage. |
||
Knowledge, attitudes and practices regarding antibiotic prescribing
On average, participants correctly answered three questions (SD = 1.33) out of a total of 8 (Table 2). Incorrect responses were most likely to appear in antibiotic therapy in bacterial pneumonia (95.52%), followed by antibiotic prescribing for upper ARIs (92.54%), ineffective antibiotics in upper ARIs (79.10%) and effective antibiotic treatment for methicillin-resistant Staphylococcus aureus (55.22%). About 30% of respondents could not determine which antibiotics cross the blood-brain barrier most effectively. On average, the antibiotic knowledge scores were not statistically different between GPs regardless of the type of health facility where they practice (p = 0.152).
| Table 2: Knowledge of respondents regarding the antibiotic prescribing | |||||
| Questions | Number (Pourcentages) of respondents giving a correct answer | p-value | |||
| Total (N=67) |
PC (n=40) |
UC (n=16) |
GRH/CS (n=11) |
||
| Antibiotics should not be prescribed for upper respiratory tract infections | 5 (7.46%) |
2 (5.00%) |
3 (18.75%) |
0 (0.00%) |
0.123 |
| Metronidazole has the best activity against anaerobes | 61 (91.04%) |
39 (97.50%) |
12 (75.00%) |
10 (90.91%) |
0.028 |
| Methicillin resistant staphylococcus aureus is resistant to beta- lactam antibiotics |
30 (44.78%) |
20 (50.00%) |
5 (31.25%) |
5 (45.45%) |
0.443 |
| Ceftriaxone most effectively crosses the blood-brain barrier | 47 (70.15%) |
30 (75.00%) |
9 (56.25%) |
8 (72.73%) |
0.375 |
| Aminoglycosides are very active if they are administered as parenteral once daily | 63 (94.03%) |
39 (97.50%) |
13 (81.25%) | 11 (100.00%) |
0.044 |
| Bacterial pneumonia (including one of the following symptoms: fast breathing, chest in-drawing or stridor) requires antibiotic treatment | 3 (4.48%) |
1 (2.50%) |
1 (6.25%) |
1 (9.09%) |
0.597 |
| Antibiotics do not reduce the duration and the occurrence of complications of upper respiratory tract infections | 14 (20.90%) |
8 (20.00%) |
3 (18.75%) |
3 (27.27%) |
0.845 |
| The average number of patients taking antibiotics should be below 30 per 100 in a primary care facility | 24 (35.82%) |
16 (40.00%) |
4 (25.00%) |
4 (36.36%) |
0.571 |
| Overall score mean (SD) | 3.69 (1.33) | 3.87 (1.18) | 3.12 (1.75) | 3.82 (0.98) | 0.152 |
| PC: Private Clinic; GRH: General Reference Hospital; HC: Health Center; SD: Standard Deviation; %: Pourcentage. | |||||
On average, respondents reported a negative attitude towards rational antibiotic prescriptions in response to the pressures of patient expectations (complacency score = - 1.78 ± 0.31) and the demands of defensive practice (fear score = - 1.67 ± 0.47). There was a relatively high level of concern about antibiotic resistance resulting from overprescribing (ignorance score = 0.50 ± 0.68). However, a lack of motivation to change antibiotic prescribing practices was evident: a negative score was shown in indifference (-0.37 ± 0.94). Respondents were convinced that the solution to antibiotic resistance was their own responsibility (responsibility avoidance score = 1.24 ± 0.74) (Table 3).
| Table 3: Attitudes and practices of respondents regarding antibiotic prescriptions | |||||
| Variable | Scores (Mean ± SD) | p - value | |||
| Total (N = 67) |
PC (n = 40) |
UC (n = 16) |
GRH/CS (n = 11) |
||
| Attitudes | |||||
| Ignorance | 0.50 ± 0.68 | 0.49 ± 0.56 | 0.39 ± 0.95 | 0.69 ± 0.63 | 0.539 |
| Complacency | -1.78 ± 0.31 | -1.78 ± 0.27 | -1.99 ± 0.39 | -1.62 ± 0.04 | 0.006 |
| Fear | -1.67 ± 0.47 | -1.70 ± 0.36 | -1.46 ± 0.73 | -1.85 ± 0.25 | 0.084 |
| Responsibility avoidance | 1.24 ± 0.74 | 1.23 ± 0.73 | 1.14 ± 0.94 | 1.45 ± 0.31 | 0.545 |
| Indifference | -0.37 ± 0.94 | -0.45 ± 0.90 | -0.33 ± 0.99 | -0.12 ± 1.08 | 0.591 |
| Pratiques | |||||
| Prescribe antibiotics in upper ARIs | 2.94 ± 1.17 | 3.50 ± 1.21 | 1.25 ± 1.11 | 3.36 ± 1.64 | 0.047 |
Respondents indicated that they would prescribe antibiotics to about 30% (SD = 11%) of children with upper ARIs. However, a relatively strong intention to reduce antibiotic prescriptions (mean score = 0.21 ± 1.29) was reported, compared to the intention to prescribe antibiotics (mean score = - 0.71 ± 0, 91). Attitudes and practices towards antibiotic prescriptions were not statistically different between physicians in different health facilities, with the exception of physicians University clinics who prescribe less antibiotics in cases of upper ARI (p = 0.047) and showed a more negative attitude towards antibiotic prescriptions in response to the pressures of patient expectations (p = 0.006) compared to physicians in other health facilities.
Associations between knowledge score with personal characteristics, attitudes and practices
After multiple linear regressions, we found that the level of knowledge was not influenced by personal characteristics of respondents. Neither age (β = 0.013; p = 0.844), nor gender (β = 0.217; p = 0.541), nor type of health facility (β = - 0.160; p = 0.476), nor clinical experience (β = 0.031; p = 0.676), nor training on antibiotic prescriptions (β = 0.135; p = 0.760) had shown any influence on the level of knowledge of antibiotics (Table 4).
| Table 4: Multiple linear regression on personal characteristics of the respondents in relation to the antibiotic knowledge score | ||||
| Variable | Coefficient | Standard error | t - value | p - value |
| Age | 0.013 | 0.066 | 0.20 | 0.844 |
| Gender | 0.217 | 0.354 | 0.61 | 0.541 |
| Health facility | -0.160 | 0.223 | -0.72 | 0.476 |
| Clinical experience | 0.031 | 0.074 | 0.42 | 0.676 |
| Training on antibiotic prescriptions | 0.135 | 0.438 | 0.31 | 0.760 |
| Constant | 2.732 | 2.055 | 1.33 | 0.189 |
We found that higher knowledge scores were associated with less responsibility avoidance when prescribing antibiotics (β = 0.919; p = 0.000). However, attitudes of complacency (β = 0.119; p = 0.820), ignorance (β = 0.294; p = 0.262), indifference (β = - 0.244; p = 0.112) and fear (β = - 0.123; p = 0.717) were not related to the level of knowledge (Table 5).
| Table 5: Multiple linear regressions on attitudes related to the antibiotic knowledge score | ||||
| Variable | Coefficient | Standard error | t - value | p - value |
| Indifference | -0.244 | 0.151 | -1.61 | 0.112 |
| Fear | -0.123 | 0.337 | -0.36 | 0.717 |
| Complacency | 0.119 | 0.521 | 0.23 | 0.820 |
| Responsibility avoidance | 0.919 | 0.236 | 3.89 | 0.000 |
| Ignorance | 0.294 | 0.259 | 1.13 | 0.262 |
| Constant | 2.315 | 0.842 | 2.75 | 0.008 |
Neither the intention to prescribe the antibiotics in case of upper ARIs (β = 0.061; p = 0.245), nor the intention to prescribe the antibiotics in outpatients (β = - 0.013; p = 0.945), nor the intention to reduce antibiotic prescriptions in outpatients (β = 0.188; p = 0.152) was not associated with level of knowledge (Table 6).
| Table 6: Multiple linear regressions on practices related to the antibiotic knowledge score. | ||||
Variable |
Coefficient | Standard error | t - value | p - value |
| Prescribe antibiotics in upper ARIs | 0.061 | 0.052 | 1.18 | 0.245 |
| Prescribe antibiotics in outpatients | -0.013 | 0.184 | -0.07 | 0.945 |
| Reduce the prescription of antibiotics in outpatients | 0.188 | 0.130 | 1.45 | 0.152 |
Constant |
3.460 | 0.263 | 13.16 | 0.000 |
To our knowledge, this is the first assessment of knowledge, attitudes and practices reported regarding antibiotic prescriptions for the treatment of ARIs in children by general practitioners working in pediatric settings in Lubumbashi.
The present study found low levels of knowledge about antibiotics in participants who got a mean of 46% correct answers on antibiotic prescriptions (3.69 out of 8 questions), compared to 55% - 86% reported in studies previous studies carried out in the DRC [20], China [12,19] and Peru [21]. This lack of knowledge is even greater in antibiotic treatments for upper ARIs: 7.46% compared to 35% to 76% reported in previous studies [19-21]. This study reported that 20.9% of respondents knew that antibiotics do not reduce the duration and occurrence of complications from upper ARIs. Only 7.46% of respondents knew that antibiotics are not indicated for the treatment of upper ARIs accompanied by fever. This correct response rate was less than 21% reported by Hoa, et al. [10]. We found that the presence of fever increased the prescription of antibiotics, although this sign does not necessarily indicate a bacterial infection [22]. It is essential to develop and implement educational programs that target the ability of healthcare professionals to accurately differentiate mild ARIs from pneumonia and that symptomatic treatment should only be given for colds [23,24]. As the Vietnamese study by Hoa, et al. [10], the present study shows that most of the respondents reported prescribing antibiotics for symptoms of upper ARIs, considering this practice to be good management of ARIs cases. Respondents to this study reported that they would prescribe antibiotics to 30% of affected patients compared to 40% in the study by Liu, et al. [12]. These misconceptions and the subsequent practice of providing antibiotics may be important factors behind the increase in antibiotic resistance [7,25]. In particular, healthcare professionals should be reminded that routinely administering antibiotics is not recommended for all febrile respiratory conditions. An unexpected and disturbing finding is that the estimated percentage of antibiotic use for cases of bacterial pneumonia was very low (4.48%). This indicates a serious lack of knowledge regarding the ability to recognize signs of pneumonia.
Attitudes can interfere with practices. We found that participants in our study reported very negative attitudes toward pressures to prescribe antibiotics to meet patient demands and expectations, and demands to prescribe antibiotics for fear of losing patients. Several studies carried out in the United Kingdom, the United States, the Netherlands and Australia had shown that complacency and fear can be the main factors prompting physicians to prescribe antibiotics [26-29], which is inconsistent with what we found in this study. As in the Chinese study by Liu, et al. [12], participants in the present study also showed a positive attitude towards recognizing the negative consequences (antibiotic resistance) of over-prescribing, although this can be further reinforced by better knowledge. Therefore, the greatest challenge may lie in the lack of motivation of physicians to change practice as indicated by the negative mean score in the attitude of indifference. Indifference can lead to low intentions to reduce antibiotic prescriptions [12]. It is a positive sign that practitioners are taking responsibility for the fight against antibiotic resistance themselves. Contrary to the results of Liu, et al. [12], participants in this study were convinced that they are responsible for antibiotic resistance. A meta-analysis by Costelloe, et al. [30] shows that the antibiotic prescriptions in primary care have contributed significantly to the development of antibiotic resistance. We believe that such a positive attitude can even lead to a reduction in antibiotic prescriptions if awareness is combined with continuing training. The campaign to reduce over-prescription of antibiotics in ARIs should take a systems approach, addressing issues related to both the knowledge and attitudes of physicians. Training programs and practice guidelines should target key knowledge gaps for prescribing physicians (eg, prescribing antibiotics for upper ARIs). More needs to be done to motivate physicians to change their prescribing behavior.
The strength of our study is the inclusion of general practitioners who provided health services to children in public and private health facilities in the city. The study fills an important gap in the literature documenting the prescriptions of antibiotics for ARI in children in Lubumbashi.
However, there are some limitations. We did not assess the knowledge and practice of general practitioners in the diagnostic process, although we do recognize that misdiagnosis can contribute to inappropriate antibiotic prescribing. The scenarios do not fully reflect the actual situation because a clinical examination could not be performed. Additionally, we relied on respondent self-report and did not assess actual practice by observing clinical performance of healthcare professionals or using a simulated client method. Attempts to generalize the results of this study should be cautious.
General practitioners treating ARIs in children in Lubumbashi have little knowledge of antibiotic prescriptions. This is marked by a high level of prescription of antibiotics for upper ARIs in particular. These GPs worry about antibiotic resistance resulting from over-prescribing antibiotics and are taking responsibility for themselves. Improved knowledge can lead to greater motivation and lead to fewer prescriptions for antibiotics.
- Liu L, Oza S, Hogan D, Perin J, Rudan I, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2014; 385: 430-440. PubMed: https://pubmed.ncbi.nlm.nih.gov/25280870/
- Biezen R, Pollack AJ, Harrison C, Brijnath B, Grando D, et al. Respiratory tract infections among children younger than 5 years: current management in Australian general practice. Med J Aust. 2015; 202 262-265. PubMed: https://pubmed.ncbi.nlm.nih.gov/25758698/
- Zhang Z, Zhan X, Zhou H, Sun F, Zhang H, et al. Antibiotic prescribing of village doctors for children under 15 years with upper respiratory tract infections in rural China: a qualitative study. Medicine. 2016; 95: e3803. PubMed: https://pubmed.ncbi.nlm.nih.gov/27281082/
- Fletcher-Lartey S, Yee M, Gaarslev C, Khan R. Why do general practitioners prescribe antibiotics for upper respiratory tract infections to meet patient expectations: a mixed methods study. BMJ Open. 206; 6. PubMed: https://pubmed.ncbi.nlm.nih.gov/27798010/
- Moro ML, Marchi M, Gagliotti C, et al. Why do paediatricians prescribe antibiotics? Results of an Italian regional project. BMC Pediatrics. 2009; 9: 69.
- Stearns CR, Gonzales R, Camargo CA Jr, et al. Antibiotic prescriptions are associated with increased patient satisfaction with emergency department visits for acute respiratory tract infections. Acad Emerg Med. 2009; 16: 934-941. PubMed: https://pubmed.ncbi.nlm.nih.gov/19799568/
- van de Sande-Bruinsma N, Grundmann H, Verloo D et al. Antimicrobial drug use and resistance in Europe. Emergency and Infectious Disease. 2008; 14: 1722–1730. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2630720/
- Fuller JD, McGeer A, Low DE. Drug-resistant pneumococcal pneumonia: clinical relevance and approach to management. Eur J Clin Microbiol Infect Dis. 2005; 24: 780–788. PubMed: https://pubmed.ncbi.nlm.nih.gov/16344922/
- WHA Resolution. WHA68.7—Global Action Plan on Antimicrobial Resistance; Sixty-Eighth World Health Assembly; WHO: Geneva, Switzerland, 2015.
- Hoa NQ, Larson M, Chuc NTK, Eriksson B, Trung NV, et al. Antibiotics and paediatric acute respiratory infections in rural Vietnam: health-care providers’ knowledge, practical competence and reported practice. Trop Med Int Health. 2009; 14: 546-555. PubMed: https://pubmed.ncbi.nlm.nih.gov/19320870/
- Petersen I, Hayward AC. Antibacterial prescribing in primary care. J Antimicrobial Chemotherapy. 2007; 60: i43–47. https://pubmed.ncbi.nlm.nih.gov/17656380/
- Liu C, Liu C, Wang D, Zhang X. Knowledge, Attitudes and Intentions to Prescribe Antibiotics: A Structural Equation Modeling Study of Primary Care Institutions in Hubei, China. Int J Environ Res Public Health. 2019; 16: 2385. PubMed: https://pubmed.ncbi.nlm.nih.gov/31284381/
- McCullough AR, Rathbone J, Parekh S, Hoffmann TC, Del Mar CB. Not in my backyard: A systematic review of clinicians’ knowledge and beliefs about antibiotic resistance. J Antimicrob Chemother. 2015; 70: 2465-2473. PubMed: https://pubmed.ncbi.nlm.nih.gov/26093375/
- USAID. Prise en charge communautaire intégrée des maladies de l’enfant: Documentation des meilleures pratiques et des goulots d'étranglement à la mise en œuvre du programme en République Démocratique du Congo. Kinshasa: USAID; 2012.
- Organisation Mondiale de la Santé, UNICEF. Manuel sur la PCIME: La prise en charge intégrée des maladies de l’enfant. Genève: OMS; 2005.
- WHO. Advocacy, Communication and Social Mobilization for TB Control: A Guide to Developing Knowledge, Attitude and Practice Surveys; World Health Organization: Geneva, Switzerland, 2008.
- Teixeira Rodrigues A, Roque F, Falcão A, Figueiras A, Herdeiro M. Understanding physician antibiotic prescribing behaviour: A systematic review of qualitative studies. Int. J. Antimicrob. Agents. 2013; 41: 203–221. PubMed: https://pubmed.ncbi.nlm.nih.gov/23127482/
- Lopez-Vazquez P, Vazquez-Lago JM, Figueiras A. Misprescription of antibiotics in primary care: A critical systematic review of its determinants. J Eval Clin .Pract 2012; 18: 473–484. PubMed: https://pubmed.ncbi.nlm.nih.gov/21210896/
- Quet F, Vlieghe E, Leyer C, Buisson Y, Newton PN, et al. Antibiotic prescription behaviours in Lao People’s Democratic Republic: A knowledge, attitude and practice survey. Bull. World Health Organ. 2015; 93: 219–227. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4431558/
- Thriemer K, Katuala Y, Batoko B, Alworonga JP, Devlieger H, et al. Antibiotic prescribing in DR Congo: A knowledge, attitude and practice survey among medical doctors and students. PLoS ONE. 2013; 8: e55495. PubMed: https://pubmed.ncbi.nlm.nih.gov/23441152/
- Garcia C, Llamocca LP, Garcia K, Jimenez A, Samalvides F, et al. Knowledge, attitudes and practice survey about antimicrobial resistance and prescribing among physicians in a hospital setting in Lima, Peru. BMC Clin Pharmacol. 2011; 11: 18. PubMed: https://pubmed.ncbi.nlm.nih.gov/22085536/
- Soon GS, Laxer RM. Approach to recurrent fever in childhood. Canadian Family Physician. 2017; 63: 756-762. PubMed: https://pubmed.ncbi.nlm.nih.gov/29025800/
- Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community‐wide campaign. JAMA. 2002; 287: 3103–3109. PubMed: https://pubmed.ncbi.nlm.nih.gov/12069673/
- Finkelstein JA, Huang SS, Kleinman K, et al. Impact of a 16‐community trial to promote judicious antibiotic use in Massachusetts. Pediatrics. 2008; 121: e15– 23. PubMed: https://pubmed.ncbi.nlm.nih.gov/18166533/
- El Khoury G, Ramia E, Salameh P. Misconceptions and malpractices toward antibiotic use in childhood upper respiratory tract infections among a cohort of Lebanese parents. Evaluation & the health professions. 2018; 41: 493-511. PubMed: https://pubmed.ncbi.nlm.nih.gov/28692318/
- Akkerman AE, Kuyvenhoven MM, van der Wouden JC, Verheij TJ. Determinants of antibiotic overprescribing in respiratory tract infections in general practice. J. Antimicrob. Chemother. 2005; 56: 930–936. PubMed: https://pubmed.ncbi.nlm.nih.gov/16155062/
- Macfarlane J, Holmes W, Macfarlane R, Britten N. Influence of patients’ expectations on antibiotic management of acute lower respiratory tract illness in general practice: Questionnaire study. BMJ 1997; 315: 1211–1214. PubMed: https://pubmed.ncbi.nlm.nih.gov/9393228/
- Cockburn J, Pit S. Prescribing behaviour in clinical practice: Patients’ expectations and doctors’ perceptions of patients’ expectations—A questionnaire study. BMJ. 1997; 315: 520–523. PubMed: https://pubmed.ncbi.nlm.nih.gov/9329308/
- Mangione-Smith R, McGlynn EA, Elliott MN, Krogstad P, Brook RH. The relationship between perceived parental expectations and pediatrician antimicrobial prescribing behavior. Pediatrics. 1999; 103: 711–718. PubMed: https://pubmed.ncbi.nlm.nih.gov/10103291/
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ. 2010; 340: 1120. PubMed: https://pubmed.ncbi.nlm.nih.gov/20483949/