Research Article
Fluticasone furoate/Vilanterol 92/22 μg once-a-day vs Beclomethasone dipropionate/Formoterol 100/6 μg b.i.d. in asthma patients: a 12-week pilot study

Claudio Terzano* and Francesca Oriolo
Respiratory Diseases Unit and School of Specialization in Respiratory Diseases Policlinico Umberto I, “Sapienza” University of Rome, Italy
*Address for Correspondence: Dr Claudio Terzano, Respiratory Diseases Unit and School of Specialization in Respiratory Diseases Policlinico Umberto I, “Sapienza” University of Rome, Italy, Tel: 0649979051; Fax: 06499790675; Email: [email protected]
Dates: Submitted: 08 September 2017; Approved: 26 September 2017; Published: 27 September 2017
How to cite this article: Terzano C, Oriolo F. Fluticasone furoate/Vilanterol 92/22 μg once-a-day vs Beclomethasone dipropionate/Formoterol 100/6 μg b.i.d. in asthma patients: a 12-week pilot study. J Pulmonol Respir Res. 2017; 1: 013-022. DOI: 10.29328/journal.jprr.1001004
Copyright License: © 2017 Terzano C, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Asthma; Asthma control test; ICS/LABA
Abstract
Two of the most recent LABA/ICS combinations for treatment of persistent asthma are Fluticasone furoate/Vilanterol 92/22 µg (Ellipta) and Beclomethasone dipropionate/Formoterol 100/6 µg (Nexthaler).
Objective: To compare once-daily Fluticasone/ Vilanterol combination with twice daily Beclomethasone/ Formoterol association in moderate asthma, in terms of quality of life and lung function.
Methods: Fourty patients with moderate asthma treated with Beclomethasone/Formoterol 100/6 µg or Fluticasone/Vilanterol 92/22 µg. We revalued patients in terms of lung function and Asthma Control Test, at 4, 8 and 12 weeks to assess any differences between the two groups. After 4 weeks, thirty-one of the fourty patients were evaluated in terms of respiratory function at predetermined time intervals.
Result: In patients treated with beclomethasone/formoterol FEV1 presented a mean value of 78% at the third visit and of 79.1% during the final check, compared with 74.5% and to 75.8% in patients in treatment with fluticasone/vilanterol (p 0.01). Mean values of IC and MMEF25-75% were higher in patients treated with beclomethasone/formoterol compared with fluticasone/vilanterol. For the dyspnea it was a difference at the third observation. For the nocturnal symptoms and the use of rescue drug there was a significant difference, except at the beginning. For the perception of control by patients, there was a difference in the two groups at the beginning, after 4 and 8 weeks. Total ACT score showed a significant difference after 4, 8 and 12 weeks. In the group treated with beclomethasone/formoterol FEV1 value was significantly higher at a distance of four hours after drug administration (p 0.04) and after the second dose (p 0.02) compared with the group treated with fluticasone/vilanterol.
Discussion: Patients in treatment with beclomethasone/formoterol showed improved asthma control and nocturnal symptoms and more stable respiratory function compared with patients receiving fluticasone/vilanterol.
Introduction
Bronchial asthma is a chronic inflammatory disease of the airways, characterized by airflow limitation, usually reversible spontaneously or after therapy, bronchial hyper-responsiveness and accelerated decline in lung function, which may progress to irreversible obstruction of the airways [1]. According to the World Health Organization asthma regards 300 million people worldwide, with prevalence data ranging from 1 to 18% in the adult population and 10-15% in children. The increase of hospitalizations and deaths due to asthma [2] justifies the importance of its correct diagnosis and therapeutic management. The underlying mechanism responsible for asthma is the excessive presence and activation of inflammatory cells within the mucosal, muscular and vascular structures of the airways, which cause the release of inflammatory mediators and the remodeling of the airways. The main symptoms of asthma are dyspnea, cough, wheezing and chest tightness; dyspnea, predominantly expiratory, occurs particularly during the night [3] due to vagal predominance and to decreased secretion of adrenaline. Cough is mainly dry, continuous or accessional; wheezing is due by the passage of air through obstructed airways; chest tightness related to bronchoconstriction.
The clinical expression of the disease varies among individuals and in the same patient over time. The severity of the disease based on the frequency of symptoms, value of forced expiratory volume in 1 second (FEV1), variability of peak expiratory flow (PEF) and quality of life. So international guidelines divides asthma in different levels of severity based on the intensity of symptoms, the degree of bronchial obstruction and the variability of respiratory function. On this basis, there are four levels of asthma severity: mild intermittent, mild persistent, moderate persistent and severe persistent.
The exacerbations of asthma are associated with symptoms worsening and with an increase of airway inflammation and are closely related to an increase of morbidity, mortality and disease management costs [4,5]. In fact, asthma is a disease with high cost for the Italian National Health Service: the main costs are indirect and due to hospitalization [6].
To evaluate asthma control we can submit questionnaires to the patient that assess in particular the frequency and intensity of diurnal and nocturnal symptoms, the use of rescue medication and the limitation in the execution of the daily and work activities: one of the most used is ACT (“Asthma Control Test”).
The purpose of therapy for asthma is maintain control of symptoms as long as possible, with attention to the safety of the treatment, its possible adverse effects and the adhesion of the patients. The therapeutic strategy includes two main categories of drugs: controller and rescue medications; the controller medications must be taken regularly to keep the disease under control, instead, the rescue medications relieve the acute bronchoconstriction and related symptoms.
Over the years, the best understanding of pathophysiological basis of asthma marked an important turning point in the treatment adding to bronchodilators, traditionally used as symptomatic therapy, anti-inflammatory drugs. Since asthma is an inflammatory disease, inhaled corticosteroids (ICS) are the most effective controller medications currently available and represent the first choice of treatment, to which long-acting beta2-agonists bronchodilators (LABA) can be added. The combination of these two categories of drugs is recommended for persistent asthma [7], to improve lung function and symptoms. LABA currently used to treat asthma, mainly formoterol and salmeterol, require dosing every 12 hours [8,9]; on the contrary vilanterol, showing a longer duration of action, requires once-daily dosing [10,11].
Two of the most recent LABA/ICS combinations for treatment of persistent asthma are Fluticasone furoate/Vilanterol 92/22 µg delivered via the Ellipta device [12-14], and Beclomethasone dipropionate/Formoterol 100/6 µg delivered via the Nexthaler device [15-17]: the former combination covers 24 h and is assumed at once-a-day regimen, the latter has to be assumed twice daily.
The aim of this study was to evaluate the effectiveness of the combination corticosteroid/β2-agonist inhaled, comparing the once-daily Fluticasone/Vilanterol combination with twice daily Beclomethasone/Formoterol association in patients with moderate asthma, in terms of quality of life, daily and nightly symptoms, use of medication bronchodilator when required and in terms of lung function.
Materials and Methods
This study was an observational analysis of asthmatic patients. We admitted to our study fourty patients with moderate persistent asthma, including 17 males and 23 females, mean age 51 years (SD: 24). Informed consent was obtained for the study, which was performed in accordance with the ethical standards of the Declaration of Helsinki. Selection criteria were patients with moderate asthma in a stable respiratory condition in the last six months before the study start, assuming Beclomethasone/Formoterol 100/6 µg or Fluticasone/Vilanterol 92/22 µg, Caucasian race, subjects of both genders>18 years of age, adherence to inhalation therapy and ability to perform spirometry. Exclusion criteria from the study were recent asthma exacerbation (within the previous six months), use of oral corticosteroid (within the previous six months) and severe comorbidities. We followed all patients for 12 weeks and they were in a clinically stable condition. At the enrollment and during the next checks, all patients completed the ACT questionnaire “Asthma Control Test”, which is useful to evaluate the level of asthma control in the four weeks before its compilation. At the enrollment all the patients were examined: anthropometric measurements (age, height and weight) were taken and pulmonary function tests, including flow/volume spirometry and N2-wash out, were conducted, using a Cosmed Quark spirometer (PFT4 SUITE- COSMED- Pavona- Italy). In particular we measured forced vital capacity (FVC), forced expiratory volume in the first second of forced and maximal exhalation (FEV1), peak expiratory flow (PEF), the mean expiratory flow between 25 and 75% of forced vital capacity (MMEF 25-75%), inspiratory capacity (IC), functional residual capacity (FRC), residual volume (RV) and total lung capacity (TLC). In all patients, we assessed the correct use of inhalers devices and the adherence to treatment.
We revalued patients, divided into two groups based on treatment, in terms of lung function and ACT, at a distance of 4, 8 and 12 weeks of observation. All patients were allowed to use salbutamol MDI 2 puffs as needed. After 4 weeks, thirty-one of the fourty patients were evaluated in terms of respiratory function at predetermined time intervals. We compared clinical and functional data to assess any significant differences between the two groups. All data are expressed as mean+/-standard deviation (SD). The statistical analysis compared the functional and clinical data obtained during visits (time 0, time +4, +8 time, time+12) in two different treatment groups. T- test for variables distributed in a normal, with the 95% CI, was applied to the analysis of mean and standard deviation values of FEV 1, FVC, IC, PEF, MMEF 25-75, FRC, RV, TLC, single items of ACT questionnaire, total ACT and, at time 0, the number of exacerbations, with a p<0.05 as statistically significant difference between the two groups.
Results
Table 1 showed the main characteristics regarding gender and age distribution of the two study groups.
| Table 1: Characteristics of gender and age distribution in the two groups. | |
| Patients treated with beclomethasone/formoterol | 10 males; 10 females mean age 51 |
| Patients treated with fluticasone/vilanterol | 7 males;13 females mean age 51 |
Table 2 shows values, expressed as mean and DS, of respiratory function parameters measured in patients receiving beclomethasone/formoterol and in patients treated with fluticasone/vilanterol at the time of enrollment.
| Table 2: Pulmonary function test in the two groups at the beginning. | |||
| Pulmonary function test | Beclomethasone/formoterol | Fluticasone/vilanterol | p-value |
| FVC% | 85.6+/-13.6 | 84.9 +/-12.06 | 0.86 |
| FEV1% | 69.6+/-6 | 68.5+/-6.4 | 0.56 |
| PEF% | 74.05+/-11.5 | 70.3+/-12.6 | 0.33 |
| MMEF25-75% | 41+/-7.3 | 34.1+/-12.3 | 0.03 |
| IC% | 88.4+/-10.2 | 80.5+/-13.7 | 0.04 |
| FRC% | 111.6+/-13.5 | 112.7 +/-14.4 | 0.80 |
| RV% | 129.4+/-16.7 | 132.1+/-15.8 | 0.60 |
| TLC% | 108.9+/-9.9 | 112.8+/-10.5 | 0.23 |
Table 3 shows values, expressed as mean and DS, of the individual components of the ACT questionnaire and its total value, recorded in the two patient groups at the beginning.
| Table 3: ACT in the two groups at the beginning. | |||
| Symptoms | Beclometasone/formoterol | Fluticasone/vilanterol | p-value |
| Daily activities limitation | 3.4+/-0.9 | 3.2+/-0.9 | 0.6 |
| Dyspnea | 2.9+/-0.9 | 2.8+/-0.9 | 0.86 |
| Nocturnal symtoms | 3+/-0.2 | 2.8+/-0.8 | 0.29 |
| Rescue medication | 3.2+/-0.9 | 3+/-0.8 | 0.47 |
| Symptoms’ control | 3.5+/-0.8 | 3+/-0.8 | 0.04 |
| Total score ACT | 16.1+/-3.2 | 15+/-3.9 | 0.31 |
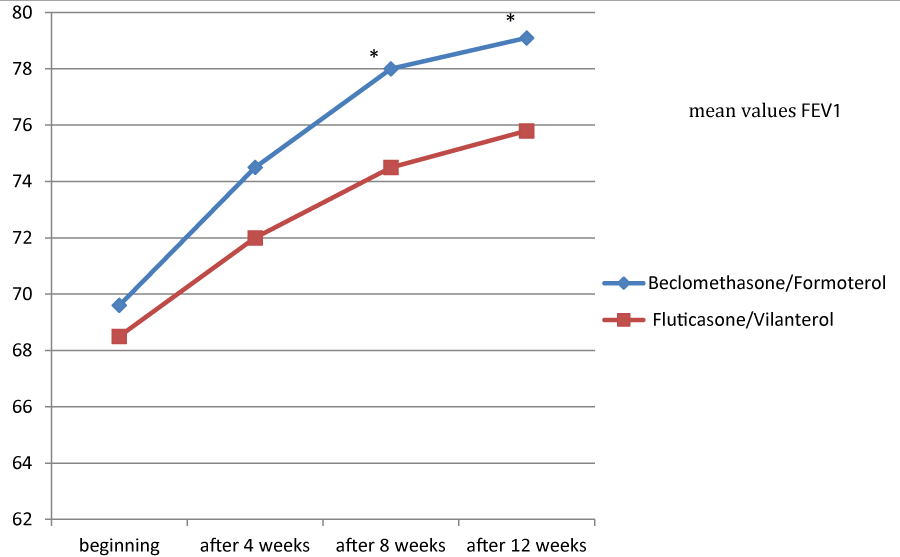
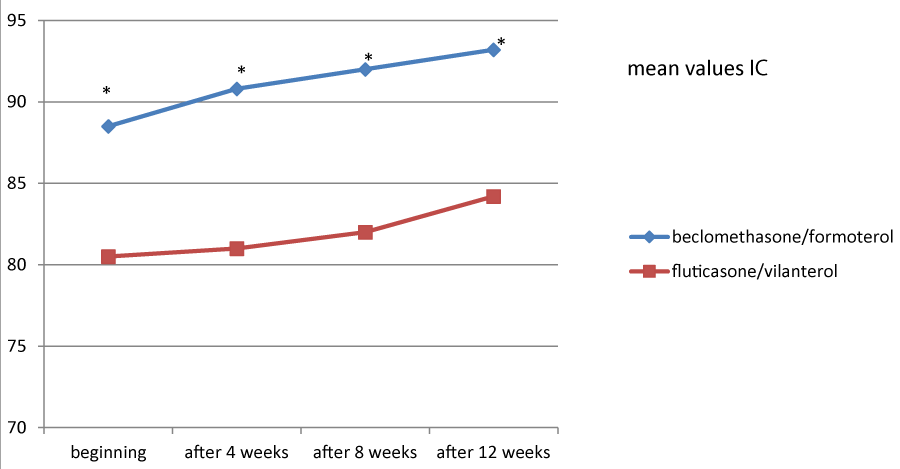
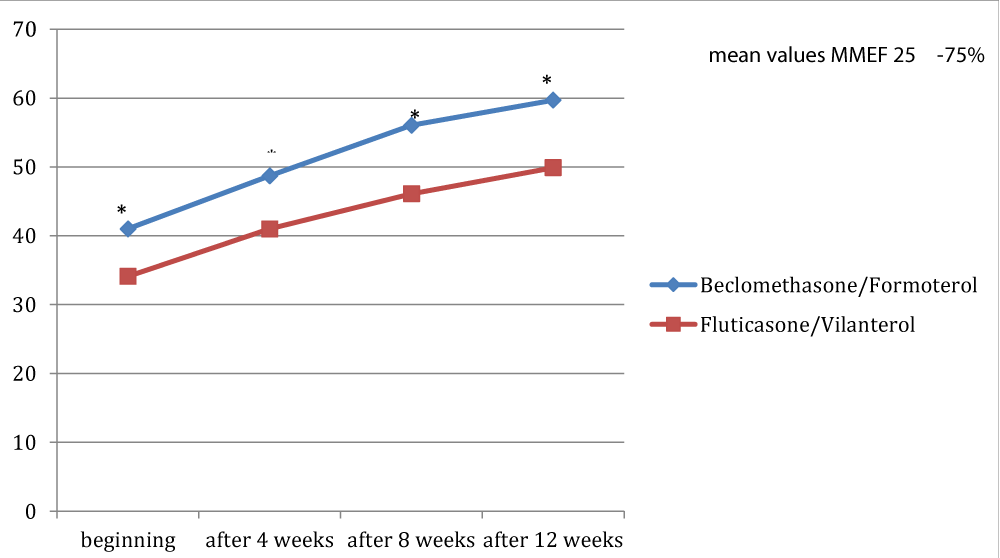
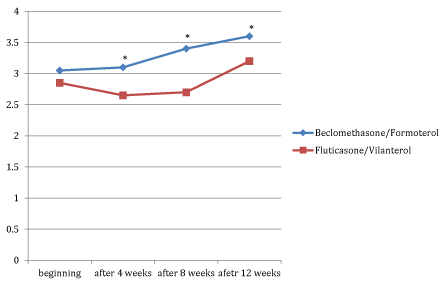
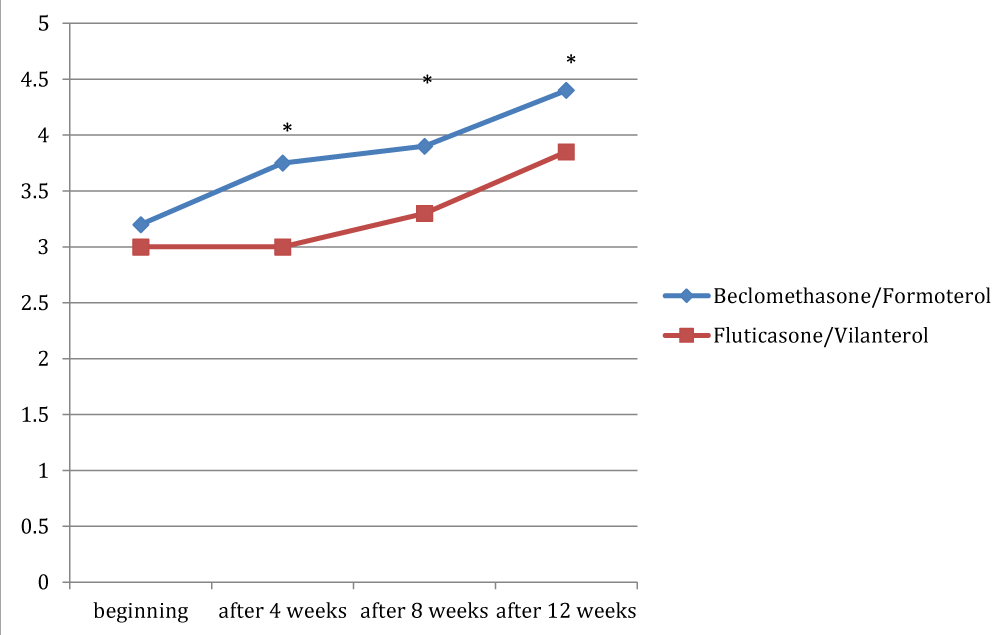
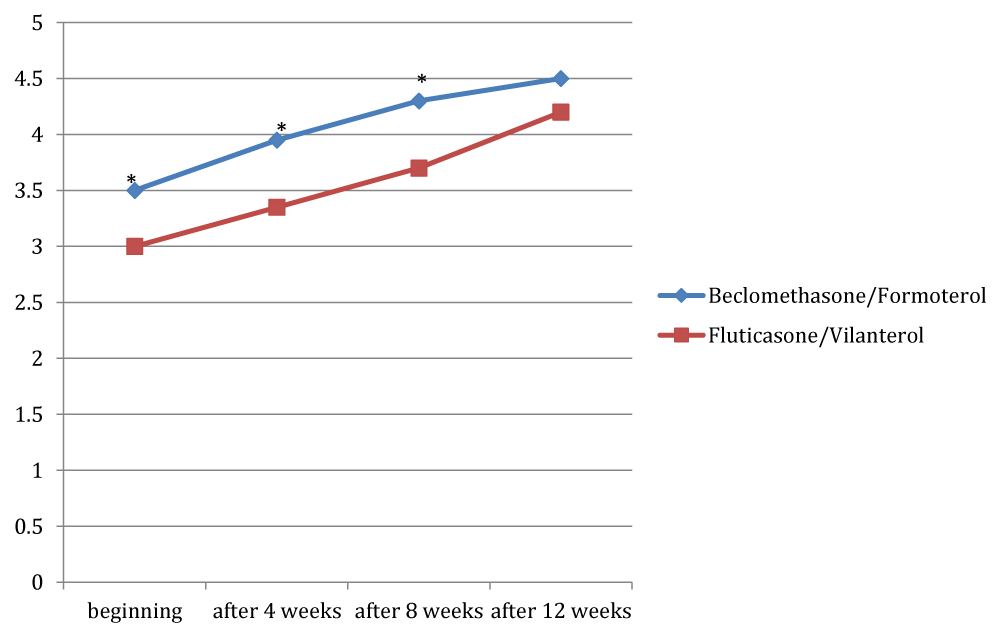
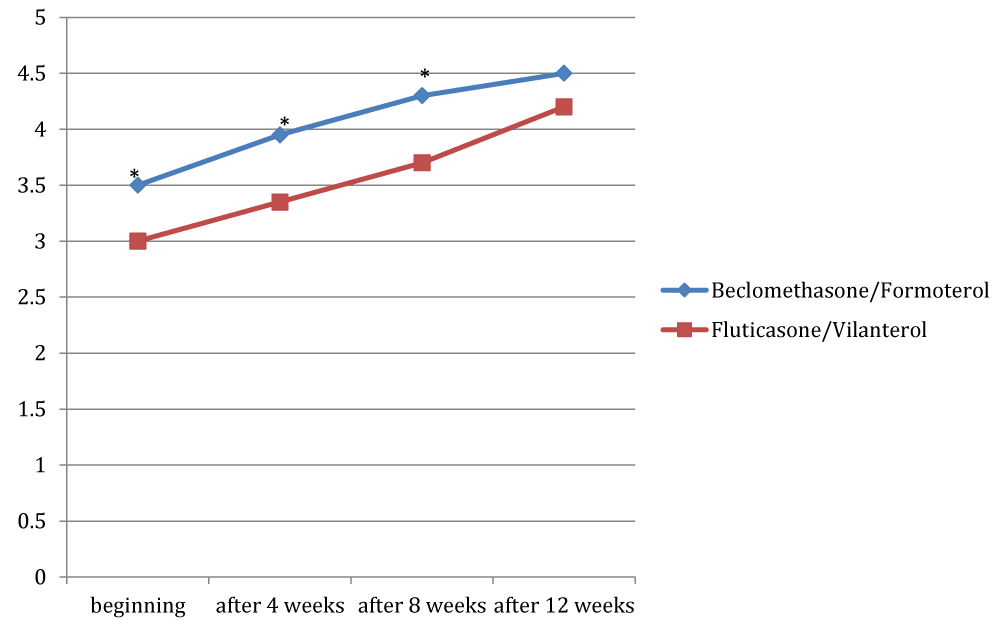
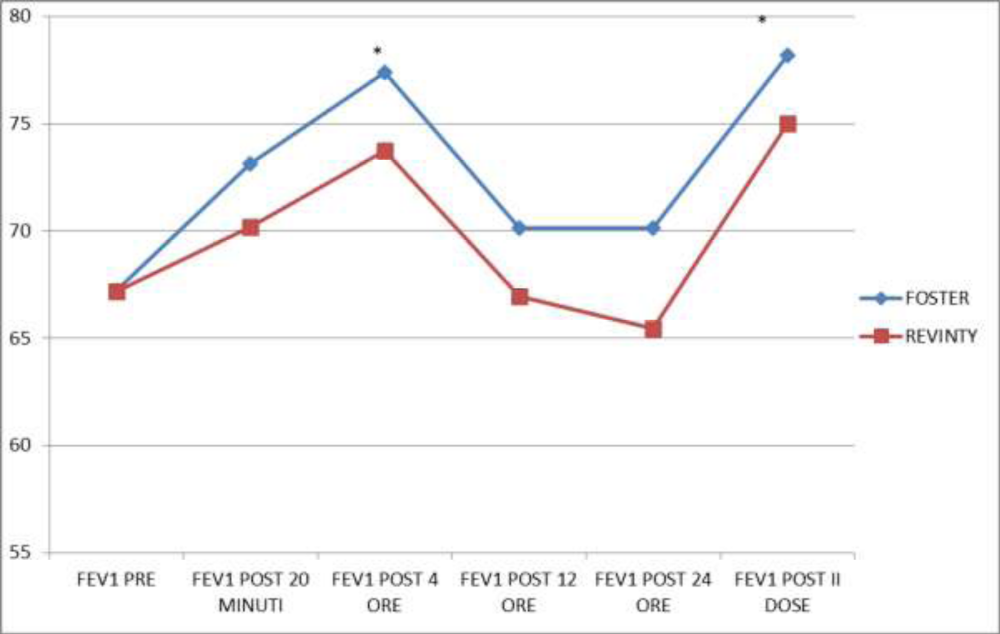
Figures 1, 2 and 3 showed the trend of the functional parameters for which there was a statistically significant difference between the two groups, which are FEV1, IC and MMEF 25-75%.
In particular, FEV1 presented in patients in treatment with beclomethason/formoterol a mean value of 78% at the third visit and of 79.1% during the final check, compared with 74.5% and to 75.8% in patients in treatment with fluticasone/vilanterol with a significant difference between the two groups (p 0.01). Mean values of IC were higher in patients treated with beclomethasone/formoterol compared with the group treated with fluticasone/vilanterol in all observations with a significant difference between the two groups (p 0.04 at time 0, p 0.01 at the time+4, p 0.008 and p 0.001 at the time+8 and at the time).Similar considerations for the trend of the mean values of MMEF25- 75%, significantly higher in patients treated with beclomethasone/formoterol, compared with the group treated with fluticasone/vilanterol (respectively p 0.03; p 0.02; p 0.003 and p 0.001).
Figures 4 to 8 showed trend of the mean values of the total ACT score and of its items, for which there was a statistically significant difference between the two groups. /p>
For the dyspnea it was a significant difference between the two groups only at the third observation, with a mean value of 3.9 in the group treated with beclomethasone/formoterol (a crisis once or twice per week) compared with a mean of 3.3 in the group receiving fluticasone/vilanterol (dyspnea reported 3-6 times a week) with a p 0.02. For the nocturnal symptoms reported by patients there was a significant difference in the two groups in all checks, except at the beginning of the study (p 0.04; p 0.003 and p 0.03 respectively).
Considering the need of assuming salbutamol, we founded significant difference in the two groups in all the observation, except at the beginning, with a p-value of 0.003 during the second visit, a p-value of 0.003 after eight weeks and a p-value of 0.005 at the end of the study. With regard to the subjective perception of symptoms control by patients, there was a significant difference in the two groups at the beginning (p 0.04), after 4 weeks (p 0.02) and after 8 weeks (p 0.006). Overall, the total ACT score showed a significant difference after 4 weeks, after 8 weeks and at the end of observation: 18.4 vs 15.8 in the time+4 ( p 0.01); 19.65 vs 17 at the time+8 (p 0.002); 21.4 (indicative of a well-controlled asthma) in the beclomethasone/formoterol group vs 19.9 in the fluticasone/vilanterol group (p 0.004).
Figure 9 showed FEV1 values in the 24 hours and in particular after 20 minutes, after four, twelve and twenty-four hours and after the second drug administration: we found that in the group treated with beclomethasone/formoterol FEV1 value was significantly higher at a distance of four hours after drug administration (p 0.04) and after the second dose (p 0.02) compared with the group treated with fluticasone/vilanterol.
Discussion
Asthma is a disease with high economic and social impact. The goal of therapy is to achieve and to maintain a good control of symptoms, and to improve lung function, reducing over time the number of exacerbations. For patients with persistent and not controlled asthma it is recommended therapy with inhaled corticosteroids and long acting β2-agonists(LABA) to improve the respiratory function and to control symptoms. LABAs currently used are formoterol and salmeterol that require administration twice daily (every 12 hours). There are many works that have demonstrated the effectiveness of formoterol/beclomethasone dipropionate, formoterol/budesonide and salmeterol/fluticasone in double administration on asthma control.
Terzano and his collaborators [8,9] evaluated in a population of patients with uncontrolled or poorly controlled asthma the reason of the lack of control and the impact of treatment on the exacerbations and on consumption of health care resources. The best control of symptoms associated with a better quality of life were in patients treated with beclomethasone/formoterol compared with patients treated with budesonide/formoterol and fluticasone/salmeterol. In our study, we founded better symptom control and respiratory function in patients treated with beclomethasone/formoterol compared with patients treated with fluticasone/vilanterol. O’Connor and others [18], in a randomized study, assessed the anti-inflammatory and dose-dipendent effect of the association beclomethasone/formoterol using non-invasive markers of inflammation, including NO and AMP: patients who were randomized to receive the drug showed a lower concentration of exhaled NO and higher FEV1, if compared with patients treated with placebo.
In a randomized double-blind study [19], the objective was to evaluate the efficacy and safety of the association beclomethasone/formoterol as maintenance therapy and as rescue medication, in a population of adult patients from moderate persistent asthma (183 centers, 14 European countries, for a period of 48 weeks). At the end of the run-in period, during which all patients were treated with the combination beclomethasone/formoterol in combination with albuterol as rescue medication, was carried randomization: some patients have been treated with the combination beclomethasone/formoterol fixed therapy and as rescue medication and others were randomized to receive salbutamol as rescue drug. The primary outcome was the time of first severe exacerbation, requiring hospitalization or treatment with oral steroid, while secondary outcomes concerned the number of severe exacerbations, improved lung function and control symptoms. In the group of patients treated with beclomethasone/formoterol therapy it was a significant increase in time of the first severe exacerbation and better symptom control. In our study, investigating at time 0 the onset of exacerbations during the six months prior to enrollment, we founded a lower occurrence of the same in the group of patients treated with beclomethason/formoterol compared with the group trated with fluticasone/vilanterol. 5 of 20 patients in the first group versus 12 of 20 patients in the second with a statistically significant difference (p 0.02). In a work of 2013, Kew and other [20] evaluated the efficacy and safety of budesonide/formoterol in a single inhaler used for maintenance and rescue therapy compared with the use of fluticasone/salmeterol or budesonide/formoterol in combination with salbutamol as rescue medication. The results obtained showed that patients treated with the combination budesonide/formoterol, both as maintenance therapy that as emergency therapy, showed a lower number of asthma exacerbations, fewer hospitalizations and a reduction in the use of oral steroids compared patients treated with SABA as needed. Similar results were found in at least three other works [21-23], in which recourse to the fixed combinations budesonide/formoterol showed efficacy not only as maintenance therapy, but also as a drug to be used as needed, improving control symptoms and lung function.
More recently it was been introduced the association fluticasone furoate/vilanterol for the treatment of persistent asthma. In phase III studies [24-26], performed on adolescents and adults with different levels of asthma not controlled, the combination of fluticasone furoate/vilanterol 100/25 or 200/25 mg once a day (approved doses in ‘EU) have more effects on lung function compared to placebo or compared to equivalent doses of fluticasone furoate or fluticasone propionate alone [27,28]. In some works [29,30], it was has been demonstrated similar efficacy between the combination fluticasone furoate/vilanterol 100/25 mg once a day and the association fluticasone propionate/salmeterol 250/50 mg twice a day to improve lung function and to reduce the risk of severe exacerbations compared with only fluticasone furoate.
Conclusion
In conclusion, more studies have shown how the treatment of persistent asthma based on the use of inhaled corticosteroid with β2- agonist to improve daytime and nighttime symptoms, quality of life and, in some cases, lung function. The results of our observational study showed that patients in the treatment group with the beclomethasone/formoterol twice-daily dosing showed improved asthma control, especially in nocturnal symptoms and in the frequency of rescue medication use, with a lower limitation of daily activities compared with patients receiving the combination fluticasone/vilanterol. In addition, in the first group we have founded, in some cases, more stable respiratory function parameters than in the second group.
References
- Sidebotham HJ, Roche WR. Asthma deaths; persistent and preventable mortality. Histopatology 2003; 43:105-117. Ref.: https://goo.gl/5E5DFj
- Levy ML The national review of asthma deaths: what did we learn and what needs to change? Breathe (Sheff). 2015; 11: 14-24. Ref.: https://goo.gl/NMjWs6
- Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, et al. Can guideline-defined asthma control be achieved?The Gaining Optimal Asthma Control Study. Am J Respir Crit Care Med. 2004; 170: 836-844. Ref.: https://goo.gl/VfuBqc
- Antonicelli L, Bucca C, Neri M, Sabbatani P, Bonifazi F, et al. Asthma severity and medical resource utilization. Eur Respir J. 2004; 23: 723-729. Ref.: https://goo.gl/L8PNF9
- King ME, Mannino DM, Holgin F. Risk factors for asthma incidence. A review of recent prospective evidence. Panminerva Med. 2004; 46: 97-110. Ref.: https://goo.gl/HffCfM
- Barnes PJ, Chung KF, Page CP. Inflammatory Mediators of Asthma: An Update Pharmacol Rev. 1998; 50: 515-596. Ref.: https://goo.gl/Y35jQi
- Barnes PJ. Cytokine modulators as novel therapies for airway disease Eur Respir J suppl. 2001; 34: 67-77. Ref.: https://goo.gl/LWU7rj
- Allegra L, Cremonesi G, Girbino G, Ingrassia E, Nicolini G, et al. Real-life prospective study on asthma control in Italy: cross-sectional phase results Respir Med. 2012; 106: 205-214. Ref.: https://goo.gl/HoS5RJ
- Terzano C, Cremonesi G, Girbino G, et al. 1-year prospective real life monitoring of asthma control and quality of life in Italy Respir Res. 2012; 6: 13-112. Ref.: https://goo.gl/j9nH2W
- Sterling R, Lim J, Frith L, et al. Efficacy and optimal dosing interval of the long-acting beta2 agonist, vilanterol, in persistent asthma: a randomized trial. Respir Med. 2012; 106: 1110-1115. Ref.: https://goo.gl/D5hnHd
- Bateman ED, Bleecker ER, Lotvall J, Woodcock A, Medley H, et al. Dose effect of once-daily fluticasone furoate in persistent asthma: a randomized trial. Respir Med. 2012; 106: 642-650. Ref.: https://goo.gl/ehb1SX
- Global initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. Report. 2014.
- Desai D, Brightling C. Cough due to asthma, cough-variant asthma and non -asthmatic eosinophilic bronchitis. Otoryngol Clin North Am. 2010; 43: 123-130. Ref.: https://goo.gl/yJRZ1t
- Kamimura M, Izumi S, Hamamoto Y, Morita A, Toyota E, et al. Superiority of nebulized corticosteroids over dry powder inhalers in certain patients with cough variant asthma or cough-predominant asthma. Allergol Int. 2012; 61: 411-417. Ref.: https://goo.gl/4BXXmG
- Colombo G, Terzano C, Colombo P, Petroianni A, Ricci A, et al. Methacholine dry powder inhaler as a new tool for bronchial challenge test. Int J Pharm. 2008; 352:165-171. Ref.: https://goo.gl/LxyKCa
- Nathan RA, Sorkness CA, Kosinsky M, Schatz M, James MS, et al. Development of the Asthma Contro Test: a survey for assessing asthma control. J allergy Clin Immunol. 2004; 113: 59-65. Ref.: https://goo.gl/Qsts7D
- Bateman ED, Boushey HA, Bousquet J, William W, Tim JM et al. The Gaining Optimal Asthma control Study American Journal Respiratory Critical Care Medicine. 2004; 170: 836-844. Ref.: https://goo.gl/VRXcKb
- Connor BJ, Collarini S, Poli G, Brindicci C, Spinola M, et al. Rapid effects of extrafine beclomethasone dipropionate/formoterol fixed combination inhaler on airway inflammation and bronchoconstriction in asthma: a randomised controlled trial. Pulm Med. 2011; 21: 11-60. Ref.: https://goo.gl/VzhPvx
- Corradi M, Pigeon-Francisco C, Baronio R, Petruzzelli S, Klaus F, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma: a double-blind, randomised controlled trial. Lancet Respir Med. 2013; 1: 23-31. Ref.: https://goo.gl/hVg1zr
- Gerzeli S, Rognoni C, Quaglini S, Cavallo MC, Papi A, et al. Cost-effectiveness and cost-utility of beclomethasone/formoterol versus fluticasone propionate/salmeterol in patients with moderate to severe asthma. Clin Drug Investig. 2012; 32: 253-265. Ref.: https://goo.gl/thdirG
- Paggiaro P, Patel S, Nicolini G, Pradelli L, Zaniolo O, et al. Stepping down from high dose fluticasone/salmeterol to extrafine BDP/F in asthma is cost-effective. Respir Med. 2013; 107: 1531-1537. Ref.: https://goo.gl/NMvmuq
- Price D, Small I, Haughney J, Ryan D, Gruffydd-Jones K, et al. Clinical and cost effectiveness of switching asthma patients from fluticasone-salmeterol to extra-fine particle beclometasone-formoterol: a retrospective matched observational study of real-world patients. Prim Care Respir J. 2013; 22: 439-448. Ref.: https://goo.gl/BK3CzQ
- Kew KM, Karner C, Mindus SM, Ferrara G. Combination formoterol and budesonide as maintenance and reliever therapy versus combination inhaler maintenance for chronic asthma in adults and children. Cochrane Database Syst Rev. 2013; 16. Ref.: https://goo.gl/pbNG8R
- Bruce SA, Scherer YK. Maintenance and symptom relief with budesonide plus formoterol reduced severe asthma exacerbations. Evidence-Based Nursing 2005; 8: 78. Ref.: https://goo.gl/C4g4Ri
- Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. American Journal of Respiratory and Critical Care Medicine 2005; 171: 129-136. Ref.: https://goo.gl/Tr2JpD
- Byrne PM, Godard P, Pistolesi M, et al. Single inhaler therapy with budesonide/formoterol improves asthma control compared with fixed dosing with budesonide/formoterol or a higher dose of budesonide alone [Abstract]. American Thoracic Society 100th International Conference. 2004; 21-26.
- Kempsford RD, Oliver A, Bal J, Tombs L, Quinn D, et al. The efficacy of once-daily fluticasone furoate/vilanterol in asthma is comparable with morning or evening dosing. Respir Med. 2013; 107: 1873-1880. Ref.: https://goo.gl/RUxz1S
- Woodcock A, Bleecker ER, Lotvall J, O'Byrne PM, Bateman ED, et al. Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: a randomized trial. Chest. 2013; 144: 1222-1229. Ref.: https://goo.gl/5tyvnP
- Byrne PM, Bleecker ER, Bateman ED, et al. Once-daily fluticasone furoate alone or combined with vilanterol in persistent asthma. Eur Respir J. 2014; 43: 773-782. Ref.: https://goo.gl/dLCZHN
- Popov TA, Petrova D, Kralimarkova TZ, Ivanov Y, Popova T, et al. Real life clinical study design supportino the effectiveness of extra-fine inhaled beclomethasone/formoterol at the level of small airways of asthmatics. Pulm Pharmacol Ther. 2013; 14: 624-629. Ref.: https://goo.gl/SNGMu2